Table of Contents |
Water is a vital chemical to life. There is no life without water. A human can go weeks without food but will die after only a few days without water. Since water is vital for life, scientists are always looking for water on celestial bodies. That is the reason scientists are so interested in finding water on Mars or the moons of Jupiter or Saturn. If there is water, there is the possibility of life.
Water (H2O) is the most abundant compound on Earth. Between 70 and 75% of the surface of the Earth is covered in water in the form of oceans, rivers, lakes, seas, bays, and other water sources. Water also makes up some of the solid parts of the Earth in the form of glaciers and polar ice. Water is also found in the gaseous portion of the Earth as water vapor in the atmosphere and in the clouds. Our planet is abundant in water and that is what made it possible for life to evolve on Earth.
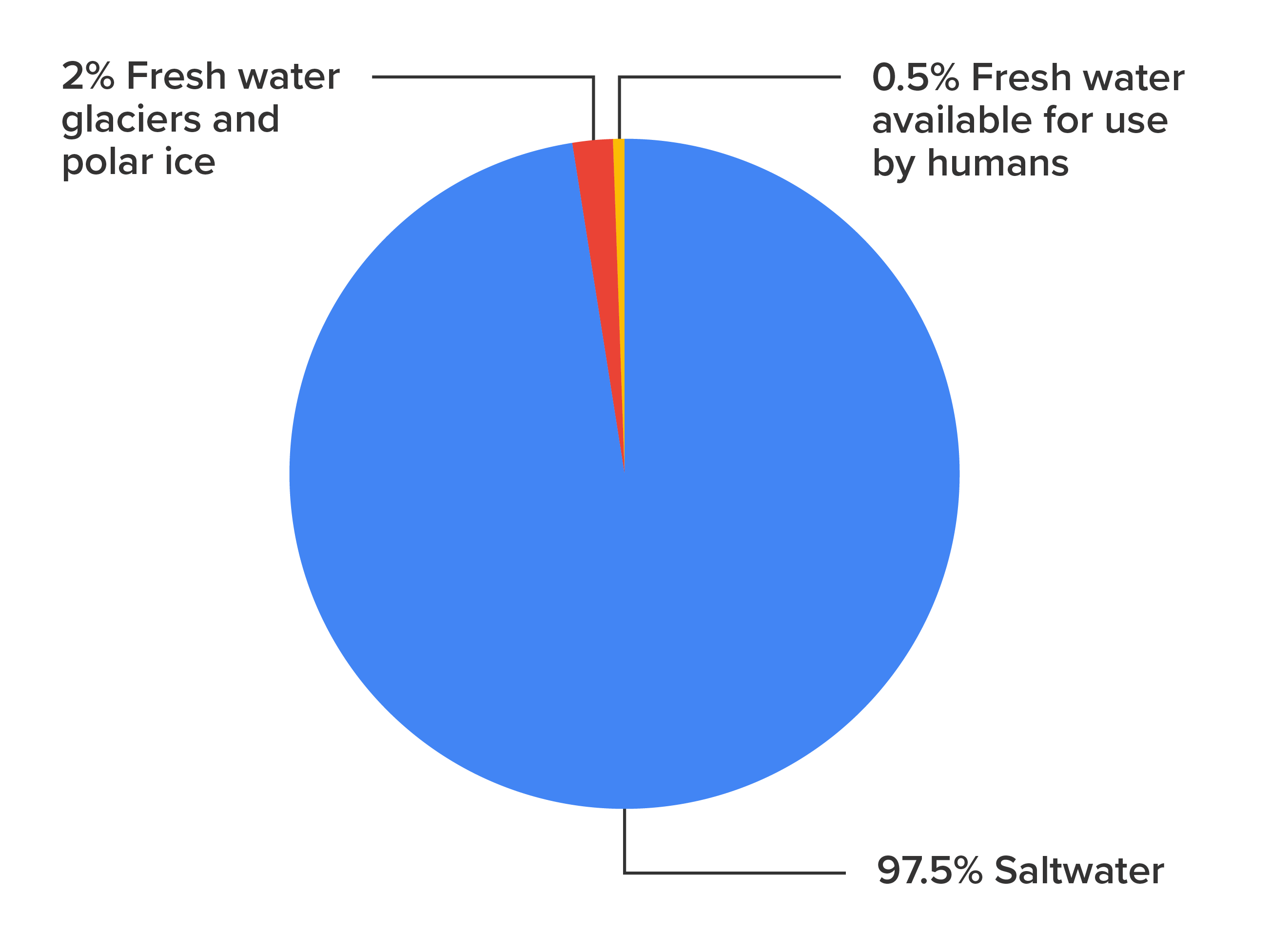
The chart below shows that water on Earth can be divided into two main categories, saltwater, and freshwater. About 97.5% of all the water on Earth is saltwater. This is mainly found in the great oceans and seas that cover most of the Earth. But you can also find saltwater marshes and lakes throughout the Earth. About 2.5% of the water on Earth is freshwater, but the majority of that is glaciers and polar ice, which makes up about 2% of the water on Earth. This means that there is only about 0.5% of all the water on Earth that is fresh water and available for use by humans.
Water is odorless, colorless, and tasteless. Any odor, color, or taste is due to substances dissolved in the water or the reflection and refraction of light off of or through the water. Water exists in three main forms. Ice is the solid form of water. Liquid water (or just water) is the liquid form of water. Water vapor is the gaseous form of water.
The melting point of water is 0°C and the boiling point is 100°C. 0°C is the transition point from solid water (ice) to liquid water (water). 100°C is the transition point from liquid water (water) to gaseous water (vapor or steam). Water has a density of 1.00 g/mL at 4°C, but as the water cools lower than 4°C, the volume expands, which lowers the density of the water. In other words, ice has a lower density than liquid water, which means ice floats on top of the water.
Pure water does not conduct electricity and is a poor conductor of heat. It is the minerals and electrolytes dissolved in water that conduct the electricity. Water has a specific heat of 1.00 J/g K, which is up to 10 times higher than the specific heat capacity of good metal conductors.
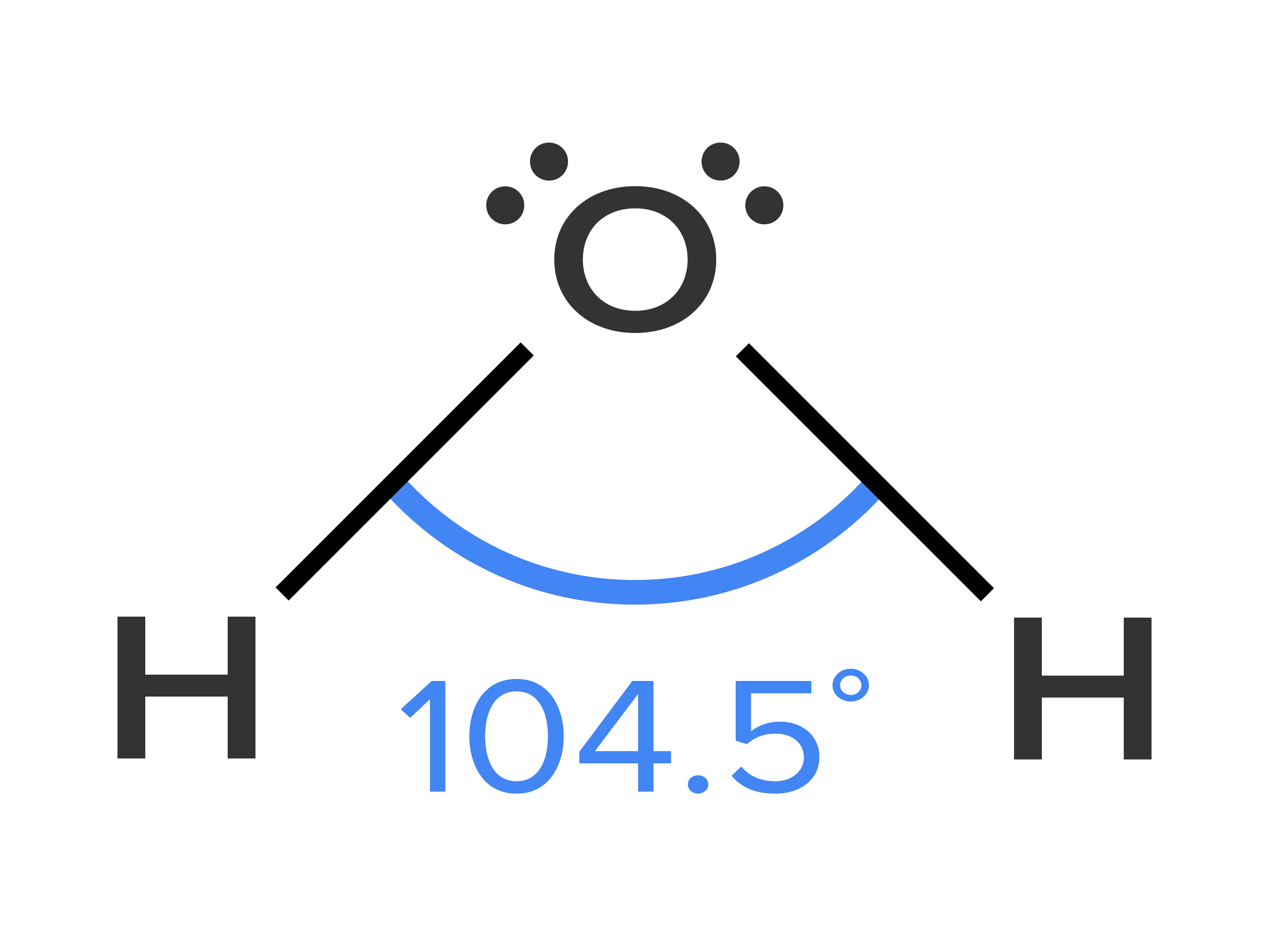
Water is composed of two atoms of hydrogen and one atom of oxygen. There are two covalent bonds in water. One covalent bond between each hydrogen and the central oxygen atom. Because oxygen has six valence electrons and two of those valence electrons are used to make the covalent bonds, the remaining four valence electrons exist as two lone pairs of electrons.
VSEPR theory states that water, with two covalent bonds and two lone pairs around the central oxygen atom, should have a version of tetrahedral geometry, known as bent geometry. The HOH bond angle is 104.45°. See the Lewis structure of water below:
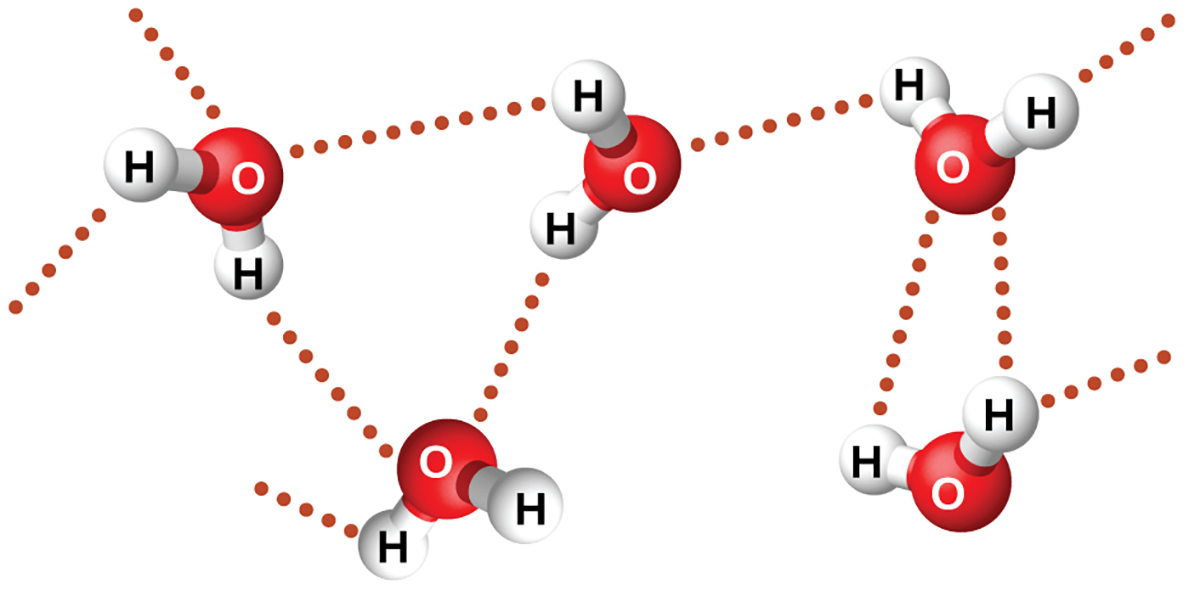
Water has a special dipole-dipole interaction called hydrogen bonding, which is a very strong intermolecular force. Because of this water has a much higher boiling point (100°C) than other molecules of similar size and shape and even a higher boiling point than many molecules that are much bigger than water.
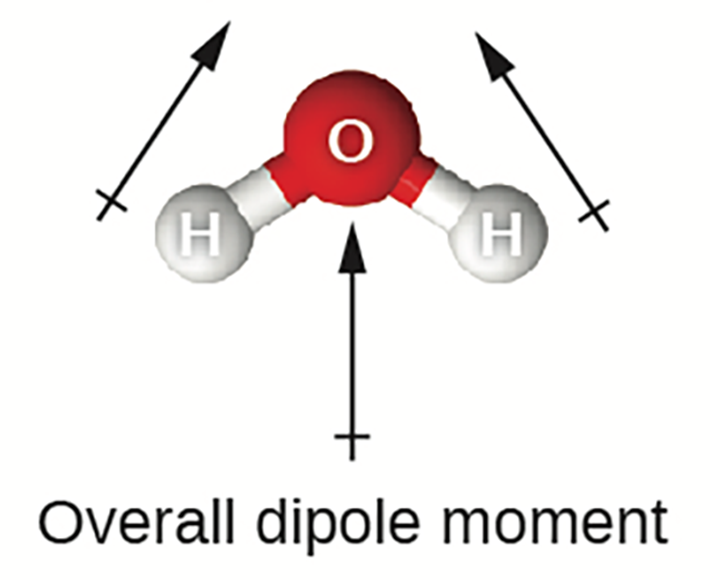
Water is a polar molecule. Water has two polar covalent bonds between each oxygen and hydrogen atom. The oxygen is more electronegative so takes on a partial negative charge compared to the hydrogen, which has a partial positive charge. Since water has a bent geometry with a bond angle of 104.45°, the two polar bonds do not cancel each other out and water has an overall dipole moment; therefore, water is a polar molecule.
When you dissolve one substance in another substance, the resulting mixture is a solution. A solution is a mixture where a solute is dissolved in a solvent. The solute is the minor component of the solution; it is what gets dissolved in the solution. The solvent is the component of the solution that does the dissolving. If the solvent of a solution is water, then the solution is an aqueous solution. The vast majority of solutions you encounter daily are aqueous solutions.
EXAMPLE
Solutions in our home would include any soft drinks, juice, bleach, glass cleaner, disinfectants, mouthwash, dishwashing liquids, liquid laundry detergents, beer, wine, liquor, and many, many more.So, why does water act as the solvent in so many of these solutions? For beverages, water is necessary as the solvent since it is not toxic. Juices, soft drinks, tea, coffee are all aqueous solutions where the solute varies. For juices, the solute is the fruit juice (such as orange, apple, and mango). For soft drinks, the solutes are sugar (or artificial sweetener), phosphoric acid, caffeine, and carbonic acid. For teas and coffee, the solutes are the chemicals extracted from tea leaves or coffee beans. These aqueous solutions are what we call potable. A potable solution is a solution that is safe to drink.
Additionally, water is the most common chemical on Earth so it makes sense that it would be found in most solutions. Also, water has the unique property that it will dissolve almost all ions, many molecules, especially polar molecules. A polar molecule is a compound that exhibits a net dipole moment. Water’s hydrogen bonding capabilities help it dissolve polar molecules. This means that many solutes are soluble or have good solubility in water.
If you remember the section on stoichiometry, solubility is the ability of a solute to be dissolved by a solvent, and to be soluble means to be able to be dissolved in a liquid. When trying to determine solubility, chemists will often use the axiom, “like dissolves like.” If you want to dissolve something, get a solvent that is like the solute.
EXAMPLE
If you want to dissolve olive oil, you would not use water because water is not “like” oil. Instead, you could use an oily liquid, like acetone or benzene, or even another oil.If you look around your house, you will find many aqueous solutions. We have already talked about all the beverages like beer, wine, liquor, fruit juices, soft drinks, teas, and coffee, which are all aqueous solutions. We also mentioned cleaners and cleansers, like bleach, glass cleaner, liquid detergents, laundry detergents, and more. But, let’s look at some other aqueous solutions you might encounter in your daily lives.
In the hospital, you will see lots of IV bags. The most common IV solution is a 0.9% NaCl solution, which means the bag has 9 grams of salt in 1000 mL of sterile water. Another IV solution is a dextrose solution, which is 5% dextrose in water. Dextrose is derived from corn and is another name for glucose. A dextrose IV will have 50 grams of glucose dissolved in 1000 mL of water.
Another common aqueous solution is vinegar, which is a 5% acetic acid solution. Acetic acid (CH3COOH) is a very common chemical and is a very weak acid. Another aqueous solution is rubbing alcohol, which is a 70% isopropyl alcohol (C3H7OH) solution dissolved in water. Hydrogen peroxide (H2O2) is a 3% aqueous solution (3% H2O2, 97% water).
IN CONTEXT
While the concentration of most solutions is reported as percentages, including beer and wine, some drinking alcohol is reported as proof instead of percentages. While most beers are around 5% alcohol and wines are about 12% alcohol, most hard liquors have a much higher percentage of alcohol. Most rums and vodkas are usually 86 proof, which is 43% alcohol. Whiskey is often around 50% alcohol or 100 proof. 151 rum is 151 proof, which is 75.5% alcohol. Pure grain alcohol is 190 proof or 95% alcohol. You should have seen a pattern that tells you the proof is 2 times the alcohol %. Therefore, 100% alcohol is 200 proof. The “alcohol” in all of these drinking alcohols is ethanol (CH3CH2OH).
In a chemistry lab, any reference to alcohol or ethanol is assumed to be 95% ethanol (which is 190 proof). If a lab experiment calls for absolute alcohol, that is referring to 100% ethanol (which is 200 proof). Absolute alcohol is often denatured. Denatured alcohol is nonpotable alcohol, which means it is not safe to drink. To get from 95% to 100% often a toxic chemical like benzene needs to be added.
As described earlier in this lesson, the two main sources of water are saltwater and freshwater. Saltwater is not potable without further purification. The purification of saltwater involves the desalination of the saltwater. Desalination means removing the salt from saltwater. The most common methods to desalinate water are distillation and reverse osmosis.
The process of purifying a liquid (separating the components of a mixture) using heating and cooling is called distillation. In distillation, impure liquid-like saltwater is placed in a glass container. The container is heated and the liquid (water) is boiled and is turned into steam. The steam rises and is carried through a condenser, which condenses the gas back to a liquid. The liquid, pure water, is then collected as freshwater.
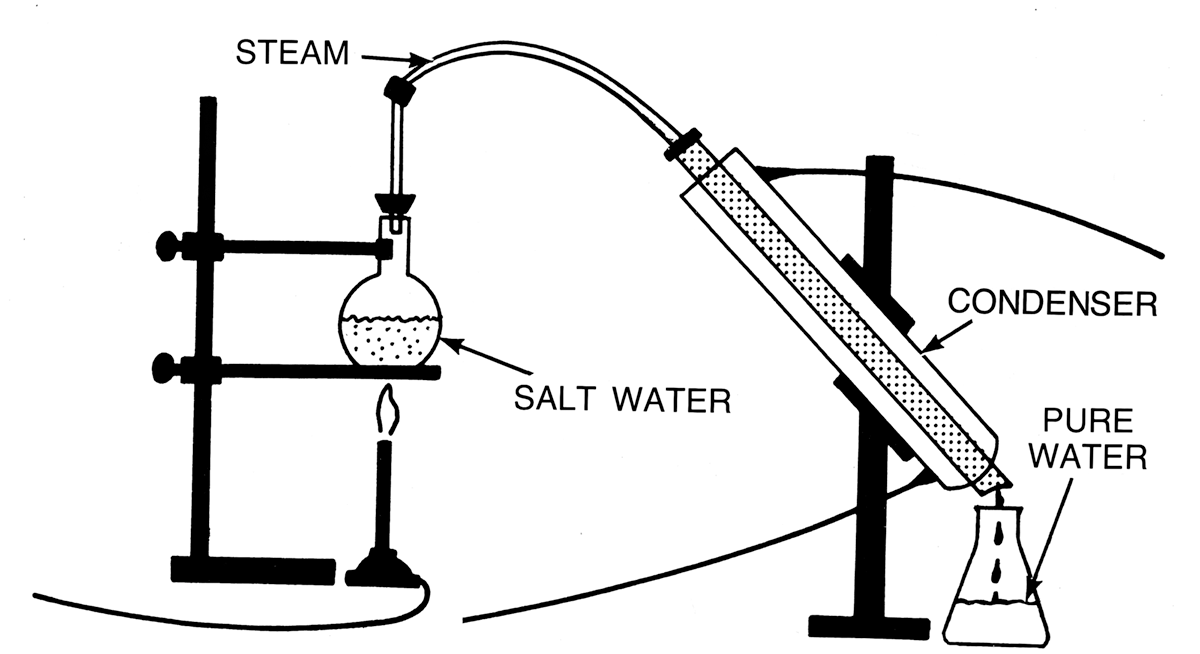
The process where a solvent moves from a lower concentration solution (freshwater) to a higher concentration solution (saltwater) is osmosis. The process in which a solvent (water) passes through a porous membrane in the opposite direction of natural osmosis when hydrostatic pressure is applied that is greater than the osmotic pressure, is referred to as reverse osmosis.
Osmosis, which is a natural process, turns freshwater into saltwater. In order to reverse this and turn salt water into fresh water, we have to apply a hydrostatic pressure to force the saltwater to move towards the freshwater, which is not the natural motion. Hydrostatic pressure is the pressure exerted by a liquid.
So, in reverse osmosis, we have to apply a hydrostatic pressure higher than the normal osmotic pressure, which will force the saltwater to pass through the membrane and create freshwater. Osmotic pressure (Π) is the pressure that would have to be applied to a pure solvent to prevent it from passing into a given solution by osmosis.
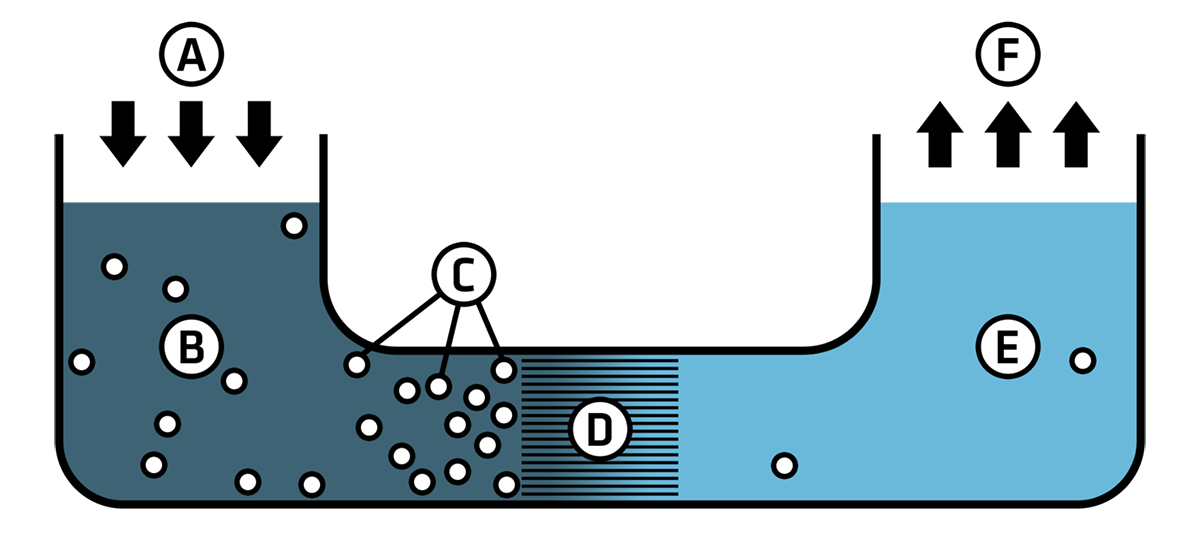
The above image shows a reverse osmosis system to create freshwater from saltwater. Pressure is applied (a) to a saltwater solution (b), which contains contaminants (c), which includes salt and other chemicals. The pressure forces the water through the semipermeable membrane (d), which generates potable water (e) and a release of any extra pressure (f).
IN CONTEXT
So, if 97.5% of the water on the Earth is salt water and if only 0.5% (of the 2.5%) of the freshwater is available to us, why do we get almost all of our water from freshwater instead of via desalination of saltwater? Some places on Earth that have very little available freshwater do use desalination. The vast majority of desalination plants to create freshwater from saltwater are found in the Middle East.
For the rest of the world, the costs of turning saltwater into freshwater are way too high to make it practical. Even though only 0.5% of the water on Earth is available freshwater, that still is a lot of water. There is enough freshwater now for our population. But freshwater is going to be a very precious resource going forwards. Many research universities and chemical companies around the world are investigating a cheap method of converting saltwater to freshwater and the hope is in the next few years, we will have developed a cheap, scalable desalination plant that produces freshwater.
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “CHEMISTRY: ATOMS FIRST 2E”. ACCESS FOR FREE AT Chemistry: Atoms First 2e. LICENSE: CREATIVE COMMONS ATTRIBUTION 4.0 INTERNATIONAL