Table of Contents |
Different pathogens produce different virulence factors, which are compounds or characteristics that contribute to their ability to cause disease. Virulence factors influence many characteristics of different diseases. For example, they affect how contagious a disease is, disease severity, and which treatment approaches will be most effective. If a virulence factor can be identified and inactivated, that may reduce the virulence of the pathogen.
Virulence factors are encoded by specific genes and these can be identified using molecular Koch’s postulates, as discussed in an earlier lesson.
In this lesson, you will learn about types of virulence factors and how they affect the activity of different pathogens.
There are varied types of virulence factors, and some are more common in certain taxa. In this lesson, we will focus on virulence factors of bacteria and viruses. After discussing types of virulence factors in general, with an emphasis on bacteria, we will focus on viral virulence factors.
The virulence factors discussed in this section can be divided into three major categories: virulence factors for adhesion to host cells; bacterial exoenzymes and toxins; and virulence factors for survival in the host and immune invasion. Toxin is a broad term that refers to biological chemicals that cause some type of harm to other organisms. In another lesson, you will learn about bacterial toxins in more detail.
The first steps in pathogenesis are exposure of the host cell to the pathogen and then adhesion of the pathogen to the cell. For a pathogen to be effective at causing infection, it must be able to adhere when the opportunity arises through exposure to a host cell.
An adhesin is a protein or glycoprotein on the surface of a pathogen (viral, bacterial, protozoal, or fungal) that attaches to receptors on a host cell. As one example, type 1 fimbrial adhesin is a molecule found on the tips of fimbriae (hairlike bristles) of enterotoxigenic Escherichia coli (ETEC). This adhesin allows the fimbriae of ETEC cells to attach to the mannose glycans found on the surface of intestinal epithelial cells.
The table below summarizes the bacterial adhesins of some important pathogens and their attachment sites. Note the relationship between the attachment site and the associated disease. Pathogens use adhesins to attach to particular receptors and this influences where they can cause infection.
| Some Bacterial Adhesins and Their Host Attachment Sites | |||
|---|---|---|---|
| Pathogen | Disease | Adhesin | Attachment Site |
| Streptococcus pyogenes | Strep throat | Protein F | Respiratory epithelial cells |
| Streptococcus mutans | Dental caries | Adhesin P1 | Teeth |
| Neisseria gonorrhoeae | Gonorrhea | Type IV pili | Urethral epithelial cells |
| ETEC | Traveler’s diarrhea | Type 1 fimbriae | Intestinal epithelial cells |
| Vibrio cholerae | Cholera | N-methylphenylalanine pili | Intestinal epithelial cells |
Following exposure and adhesion, the next step in pathogenesis is invasion. Pathogens need to penetrate cells to enter the body. In many cases, pathogens achieve invasion by entering the bloodstream, and this allows them to spread effectively through the body. Chemicals produced by pathogens both aid in their spread and contribute to signs and symptoms of disease.
Pathogens often produce exoenzymes (extracellular enzymes) that help them pass through tissues to invade. These exoenzymes target particular tissues and can facilitate invasion, support growth, or help the pathogen evade the immune system.
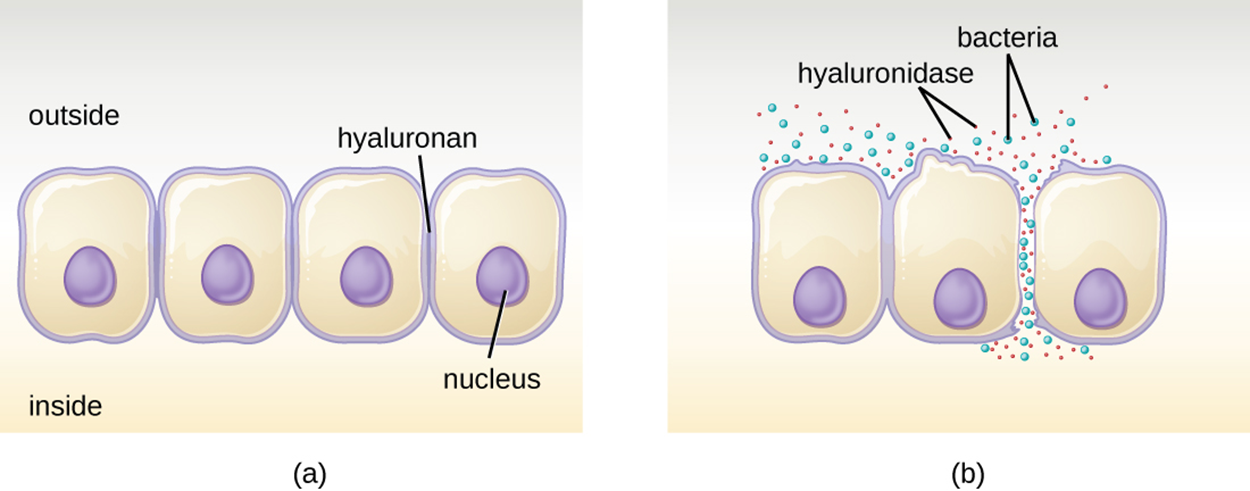
For example, as shown in the image below, hyaluronidase is an enzyme produced by multiple pathogens (including Staphylococcus aureus) that degrades the glucoside hyaluronan (hyaluronic acid) between adjacent cells. This allows the pathogen to pass between cells that would otherwise be too tightly joined together. Part (a) of the image shows intact cells, and part (b) shows how bacteria can move between cells when hyaluronidase degrades hyaluronan, which holds cells together.
Nucleases are another type of exoenzyme. These enzymes degrade extracellular DNA that is released as cells die and could otherwise trap bacteria.
Phospholipases degrade phospholipids in cell membranes, helping to allow entry to cells or helping bacteria escape from membrane-bound compartments within cells. For example, phagocytes sometimes engulf bacteria in a phagosome and phospholipases allow some bacteria to escape from the phagosome.
Some pathogens produce proteases, which are enzymes that break down proteins. These are classified based on their substrate target or on whether they contain metal in the active site of the enzyme.
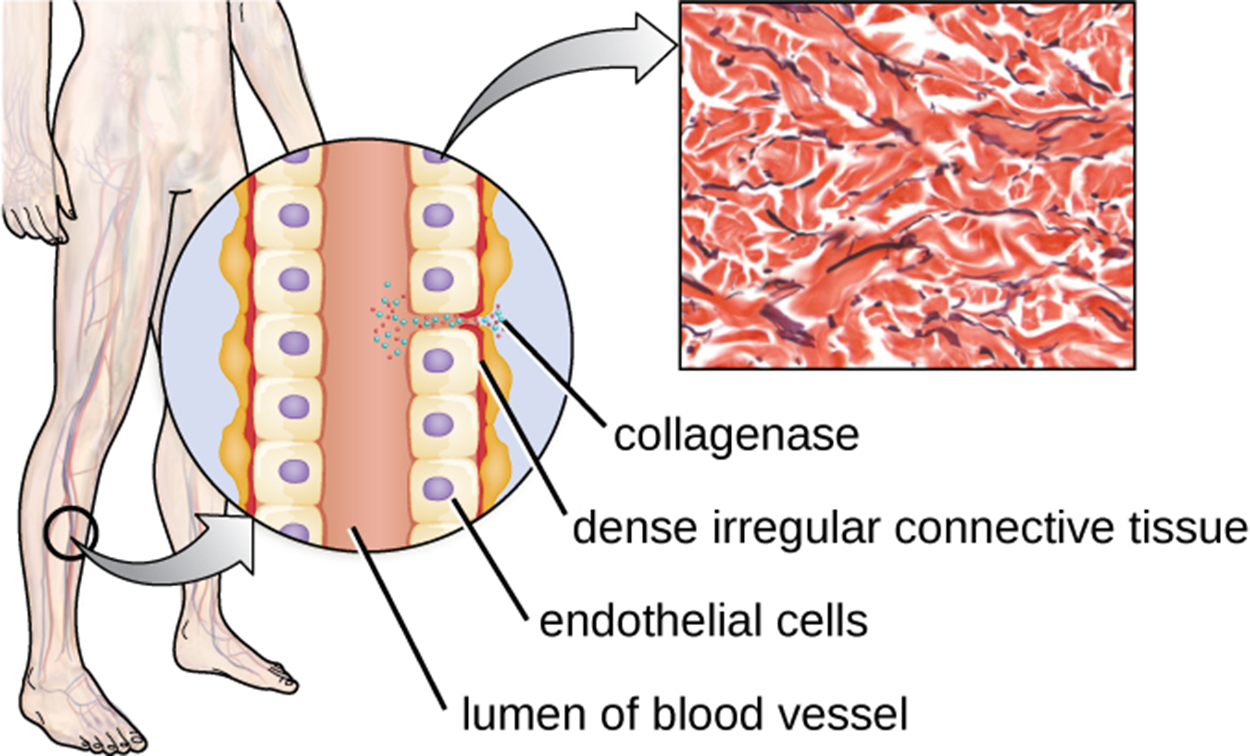
Collagenase is a protease that contains a metal ion. By breaking down collagen (an important component of connective tissue), this enzyme helps pathogens penetrate and spread through tissue. The image below shows how collagenase can provide an opening between cells, allowing pathogens to move through the endothelial cells of a blood vessel so that they can spread through the bloodstream.
The table below shows examples of classes of exoenzymes and their functions, showing how they can aid in pathogenesis.
| Some Classes of Exoenzymes and Their Targets | ||
|---|---|---|
| Class | Example | Function |
| Glycohydrolases | Hyaluronidase in S. aureus | Degrades hyaluronic acid that cements cells together to promote spreading through tissues |
| Nucleases | DNase produced by S. aureus | Degrades DNA released by dying cells (bacteria and host cells) that can trap the bacteria, thus promoting spread |
| Phospholipases | Phospholipase C of Bacillus anthracis | Degrades phospholipid bilayer of host cells, causing cellular lysis, and degrades membrane of phagosomes to enable escape into the cytoplasm |
| Proteases | Collagenase in Clostridium perfringens | Degrades collagen in connective tissue to promote spread |
As mentioned above, collagenase and other virulence factors can help bacteria enter the blood so that they can spread through the bloodstream. The table below summarizes terms used to describe different types of pathogens in the blood (-emia refers to blood).
| Type of Pathogen/Compound | Term |
|---|---|
| Viruses | Viremia |
| Bacteria in the blood | Bacteremia |
| Bacteria present and multiplying in the blood | Septicemia |
| Toxins | Toxemia |
Note that septicemia causes sepsis, and affected patients are described as septic. Sepsis can cause shock, a life-threatening decrease in blood pressure that prevents cells and tissues from receiving enough oxygen and nutrients. Some bacteria have virulence factors that contribute to shock. For example, they may produce toxins that cause tissue damage that results in low blood pressure. Gram-negative bacteria are engulfed by phagocytes that release tumor necrosis factor, which can produce inflammation and fever. Tumor necrosis factor also binds to blood vessels to increase their permeability, allowing fluids to move from the blood into the tissues. This can potentially produce a severe inflammatory response and excessively low amount of body fluid, leading to low blood pressure that can possibly lead to multi-organ failure, shock, and death.
Some virulence factors are used to evade the immune system. For example, many bacteria are able to avoid phagocytosis by the immune system.
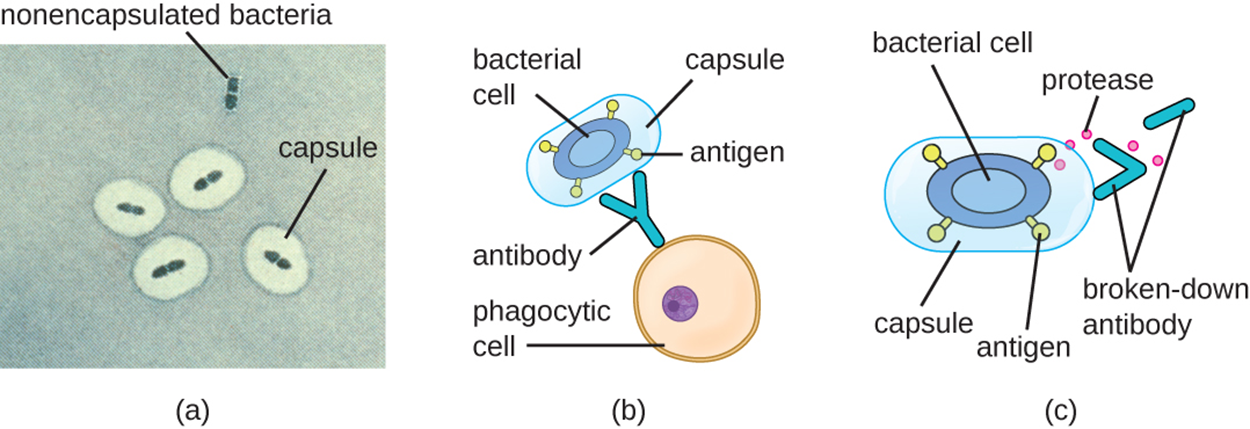
The image below shows how bacterial cells can avoid recognition by the immune system using capsules and proteases. Capsules serve other functions, but can also help by shielding antigens on the surface of the bacterial cell from recognition by antibodies. The immune system can recognize various antigens on the surface of bacterial cells when those antigens are exposed. Part (a) shows bacteria in capsules and also an example of a nonencapsulated bacterium. Part (b) shows a phagocytic cell approaching a bacterial cell surrounded by a capsule that encloses its antigens, meaning that the antibody cannot bind to the antigens. Note also that the capsule makes bacteria larger, which also makes them harder to engulf. Part (c) shows how a protease can break down antibodies, preventing them from binding to bacterial antigens.
Some bacteria use other virulence factors to evade the immune system. For example, the causative agent of tuberculosis (Mycobacterium tuberculosis) has mycolic acid in its cell envelope that helps the bacterium resist being killed if it is engulfed by a phagocyte.
Most strains of S. aureus produce coagulase, which triggers a cascade of events related to blood clotting. As a result, the clot shields the bacteria from phagocytic cells.
In contrast, kinases trigger a series of events that result in the digestion of clots and release S. aureus to spread if conditions require it (e.g., if there is a lack of nutrient supply available within the clot).
A very different mechanism of evading the immune system is antigenic variation. When surface antigens change sufficiently, the immune system does not recognize them. Some bacteria have mechanisms to promote antigenic variation. These include the bacterium that causes Lyme disease (Borrelia burgdorferi) and the bacterium that causes gonorrhea (N. gonorrhoeae).
Viral pathogens have some similarities to bacteria in their virulence factors, although they also have important differences. For example, viral pathogens have adhesins and also use antigenic variation.
As with bacteria, viruses must adhere to cells so that they can invade. Adhesins that are part of the viral capsid or envelope bind to specific receptors, determining which cells the virus can invade. This is why specific viruses bind to particular tissues.
The table below summarizes examples of viral adhesins and their attachment sites. As with bacteria, note the relationship between the attachment site and the type of disease caused.
| Some Viral Adhesins and Their Host Attachment Sites | |||
|---|---|---|---|
| Pathogen | Disease | Adhesin | Attachment Site |
| Influenza virus | Influenza | Hemagglutinin | Sialic acid of respiratory and intestinal cells |
| Herpes simplex virus I or II | Oral herpes, genital herpes | Glycoproteins gB, gC, gD | Heparan sulfate on mucosal surfaces of the mouth and genitals |
| Human immunodeficiency virus | HIV/AIDS | Glycoprotein gp120 | CD4 and CCR5 or CXCR4 of immune system cells |
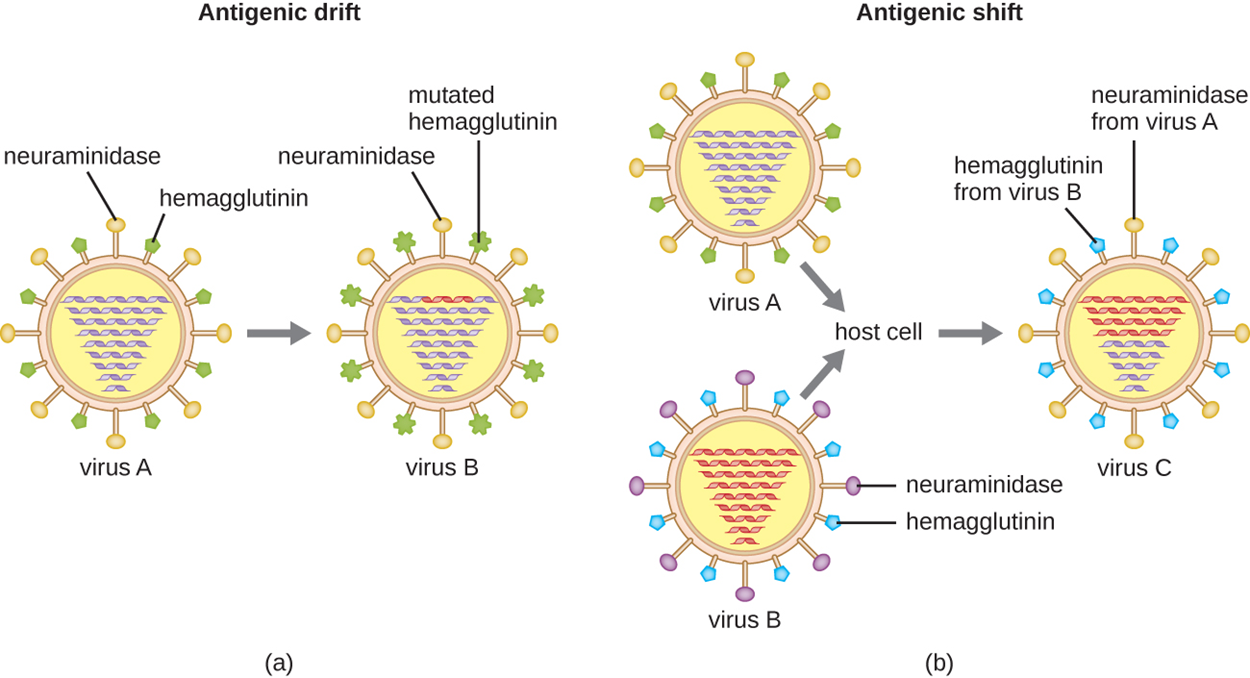
Some viruses can also use antigenic variation. The influenza virus is a good example of this and exhibits two types of antigenic variation: antigenic drift and antigenic shift. Antigenic drift (shown in part (a) of the image below) occurs because of mutations that cause relatively small changes in the influenza viral spike proteins neuraminidase and hemagglutinin. Antigenic shift is a much larger genetic change due to viral reassortment that typically occurs when two different viral strains infect the same host and produce new viruses with characteristics of each. Part (b) of the image shows how antigenic shift can produce a virus with surface proteins from different viruses.
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “MICROBIOLOGY.” ACCESS FOR FREE AT openstax.org/details/books/microbiology. LICENSE: CC ATTRIBUTION 4.0 INTERNATIONAL.