Table of Contents |
There are many types of light microscopes, which use light to visualize images. Early microscopes used light, and light microscopes are still widely used. However, different types of light microscopes have been developed to address specialized needs. In particular, many microorganisms are almost translucent, and different types of light microscopes increase contrast. It is valuable to understand the advantages and disadvantages of different types of microscopes.
The wavelengths of visible light limit the maximum theoretical resolution of light microscopes to 1,000x or sometimes 1,500x. For higher magnifications, other microscope types are needed.
Brightfield microscopes are widely used and produce a dark image on a bright background. Light passes through an objective lens and an ocular lens. As it passes through the specimen, the parts of the structure differentially transmit, absorb, reflect, and refract light. The parts and magnification of brightfield microscopes are discussed in Observing the Microscopic World.
An important limitation of brightfield microscopes is that stains are often needed to increase contrast and many stains kill living organisms. Therefore, brightfield microscopes may not be the best choice to view motility (movement) in many types of living microbes. Darkfield microscopes are similar to brightfield microscopes, but as the image below illustrates, they have an opaque light stop between the illuminator and the condenser lens that blocks the center of the beam of light. A hollow cone of light is focused on the specimen. As a result, the background is generally dark, and the specimen is illuminated by light that is refracted or reflected by the specimen. This increases the contrast, making it easier to view translucent specimens without needing stains.
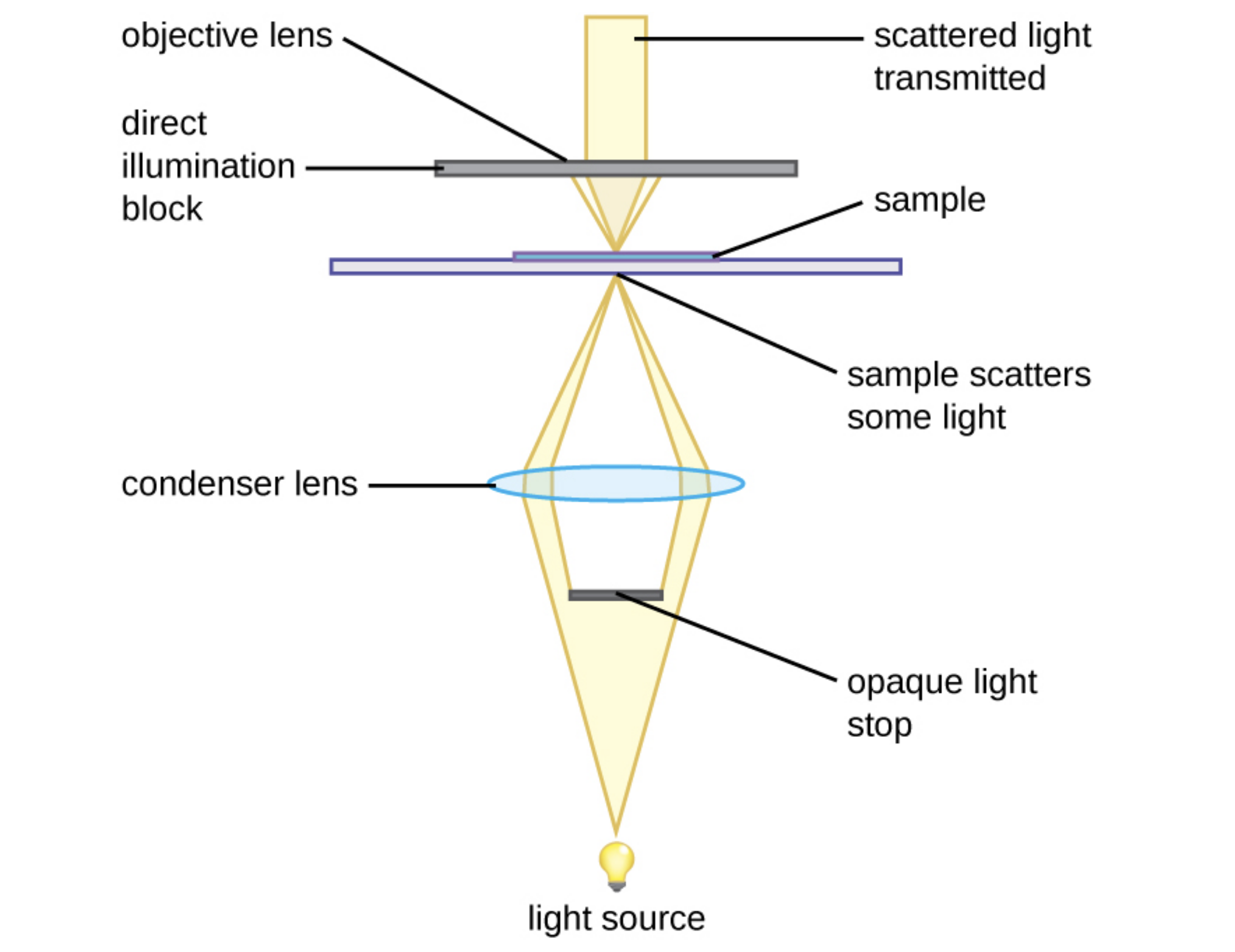
The use of a darkfield microscope allows us to view living, unstained samples, such as in the image below of the spirochete Treponema pallidum. Similar to a photographic negative, the spirochetes appear bright against a dark background.
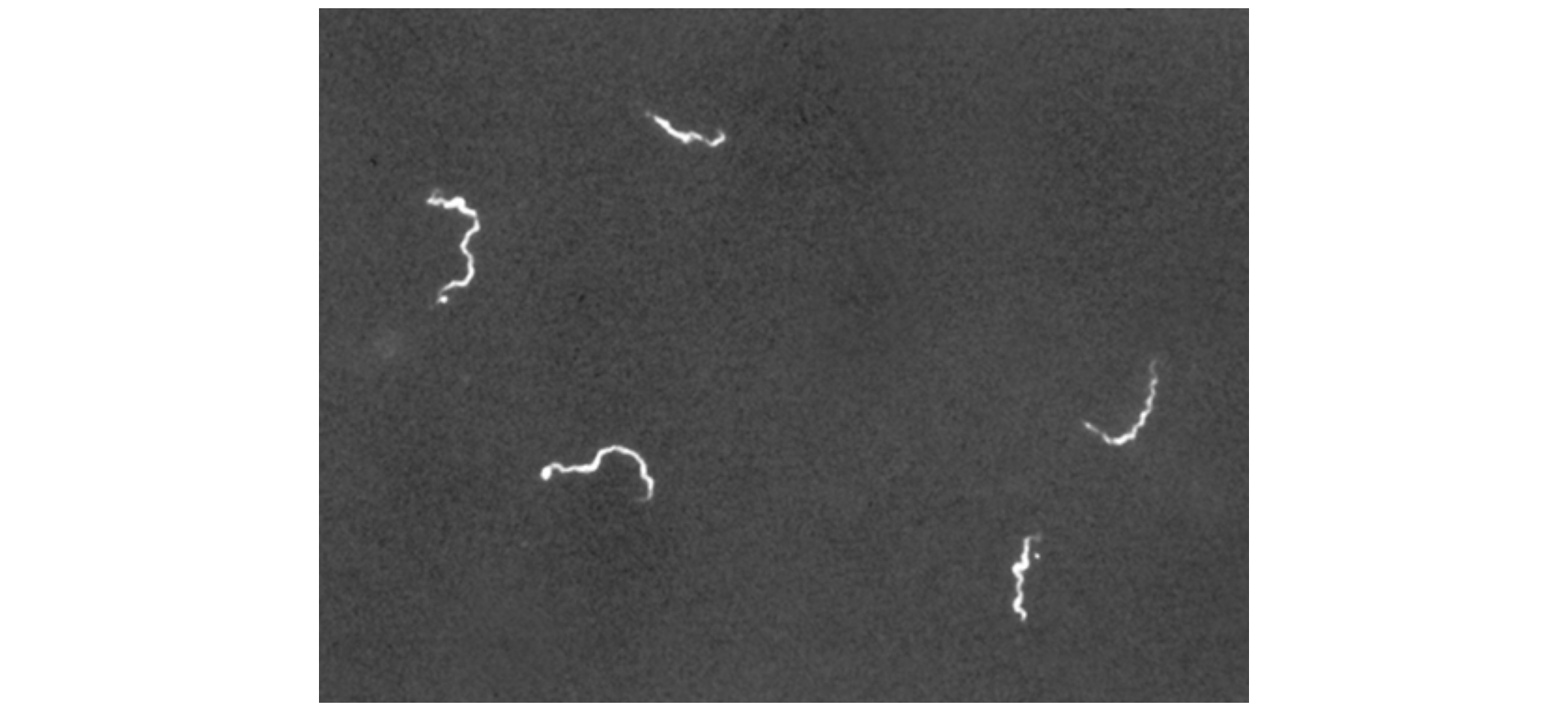
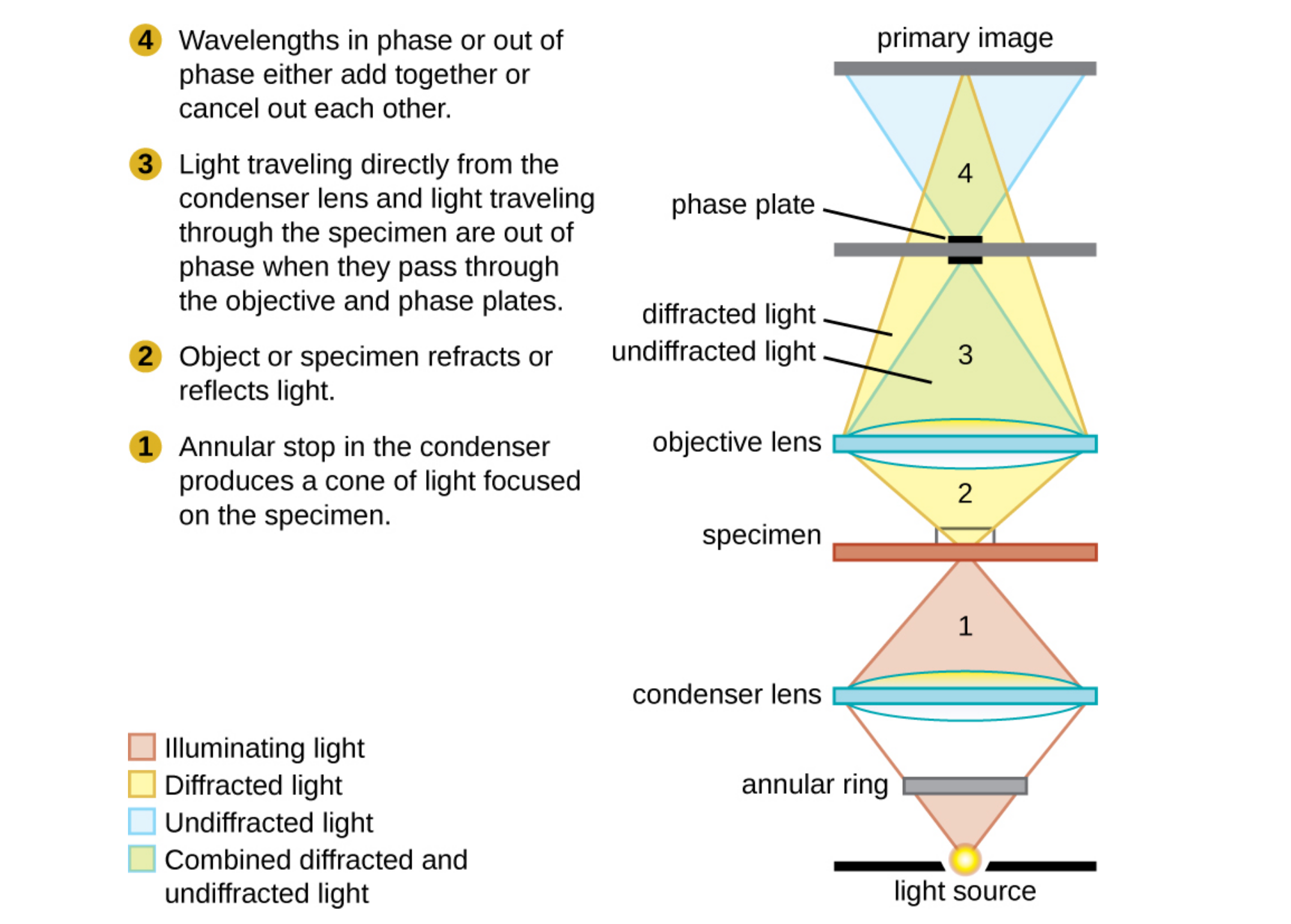
Another way to improve contrast without staining is by using a phase-contrast microscope. This type of microscope has an annular stop in the condenser that produces a hollow cone of light that is focused on the specimen before reaching the objective. The objective contains a phase plate containing a phase ring. As a result, light traveling directly from the illuminator passes through the phase ring while light refracted or reflected by the specimen passes through the plate. This causes waves traveling through the ring to be about one half of a wavelength out of phase with those passing through the plate.
Because waves have peaks and troughs, they can add together (if in phase together) or cancel each other out (if out of phase). When the wavelengths are out of phase, wave troughs will cancel out wave peaks (destructive interference). In a phase-contrast microscope, light waves traveling through the phase ring are about one-half of a wavelength out of phase with those passing through the plate. Structures that refract light then appear dark against a bright background of only unrefracted light. More generally, structures that differ in features such as refractive index will differ in levels of darkness.
The diagram below is of a phase-contrast microscope. It illustrates phase differences between light passing through the object and background. These differences are produced by passing the rays through different parts of a phase plate. The light rays are superimposed in the image plane, producing contrast because of their interference.
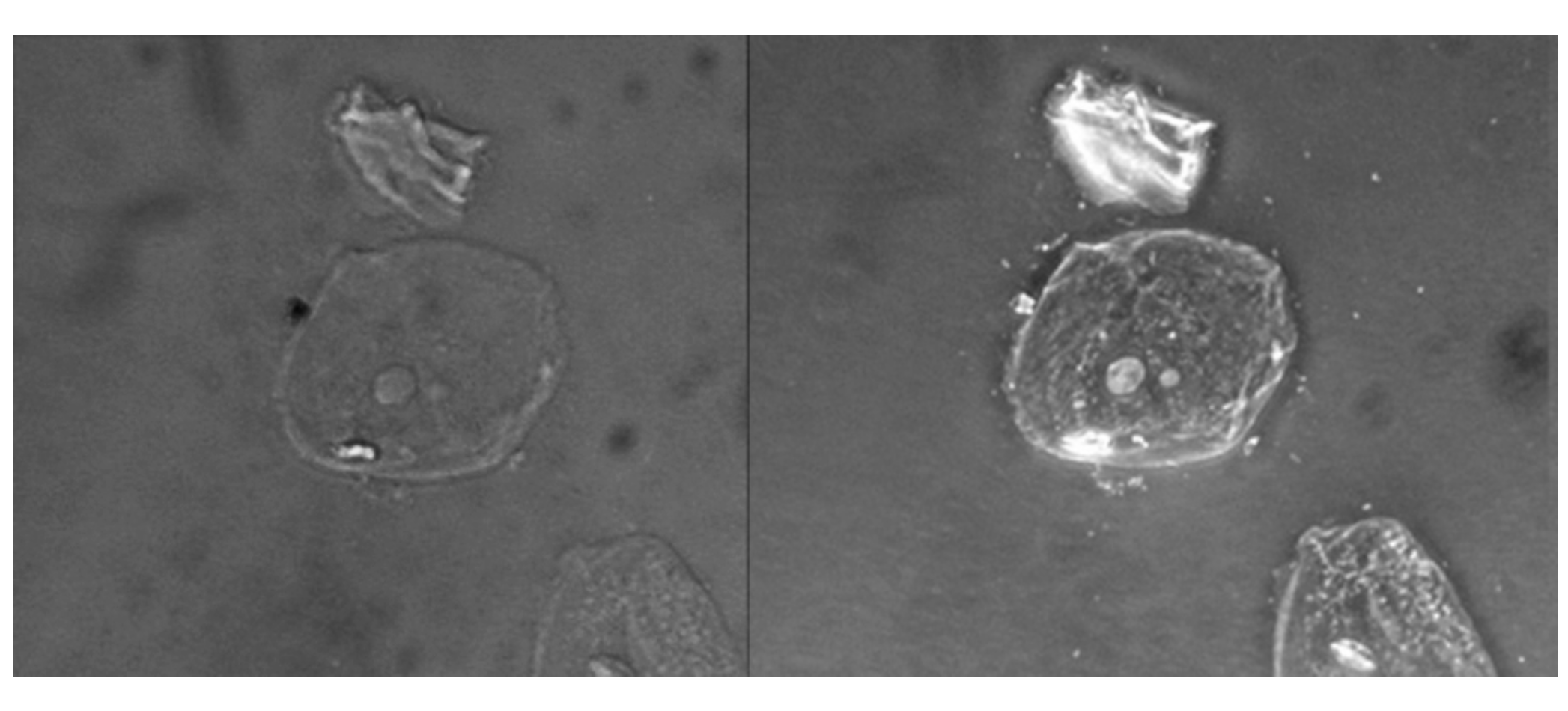
You can see below a comparison of a brightfield image (left) with a phase-contrast image (right) of the same unstained simple squamous epithelial cells. The cells are in the center and bottom right of each photograph (the irregular item above the cells is acellular debris). Notice that the unstained cells in the brightfield image are almost invisible against the background, whereas the cells in the phase-contrast image appear to glow against the background, revealing far more detail.
Differential interference contrast (DIC) microscopes also help to increase contrast to distinguish structures within live, unstained specimens. They are similar to phase-contrast microscopes in that they use interference patterns to enhance contrast between different features of a specimen. In a DIC microscope, two beams of light are created in which the direction of wave movement (polarization) differs. Once the beams pass through either the specimen or specimen-free space, they are recombined, and the effects of the specimens cause differences in the interference patterns generated by the combining of the beams. This results in high-contrast images of living organisms with a three-dimensional structure.
Below is a DIC image of Fonsecaea pedrosoi grown on modified Leonian’s agar. This fungus causes chromoblastomycosis, a chronic skin infection common in tropical and subtropical climates.

Fluorescence microscopes use fluorescent chromophores called fluorochromes (fluorophore). These fluorochromes, which can be natural substances or stains, absorb energy from a light source and then release it.
The microscope transmits an excitation light, generally a form of short-wavelength electromagnetic radiation, toward the specimen. The chromophores absorb the excitation light and emit visible light with longer wavelengths. The excitation light is then filtered out (in part because ultraviolet light is harmful to the eyes) so that only visible light passes through the ocular lens. This produces an image of the specimen in bright colors against a dark background.
Fluorescence microscopes are particularly useful in clinical microbiology. They can be used to identify pathogens, to find particular species within an environment, or to find the locations of particular molecules and structures within a cell. Approaches have also been developed to distinguish living from dead cells based on whether they take up particular fluorochromes. Multiple fluorochromes can be used on the same specimen to show different structures or features.
EXAMPLE
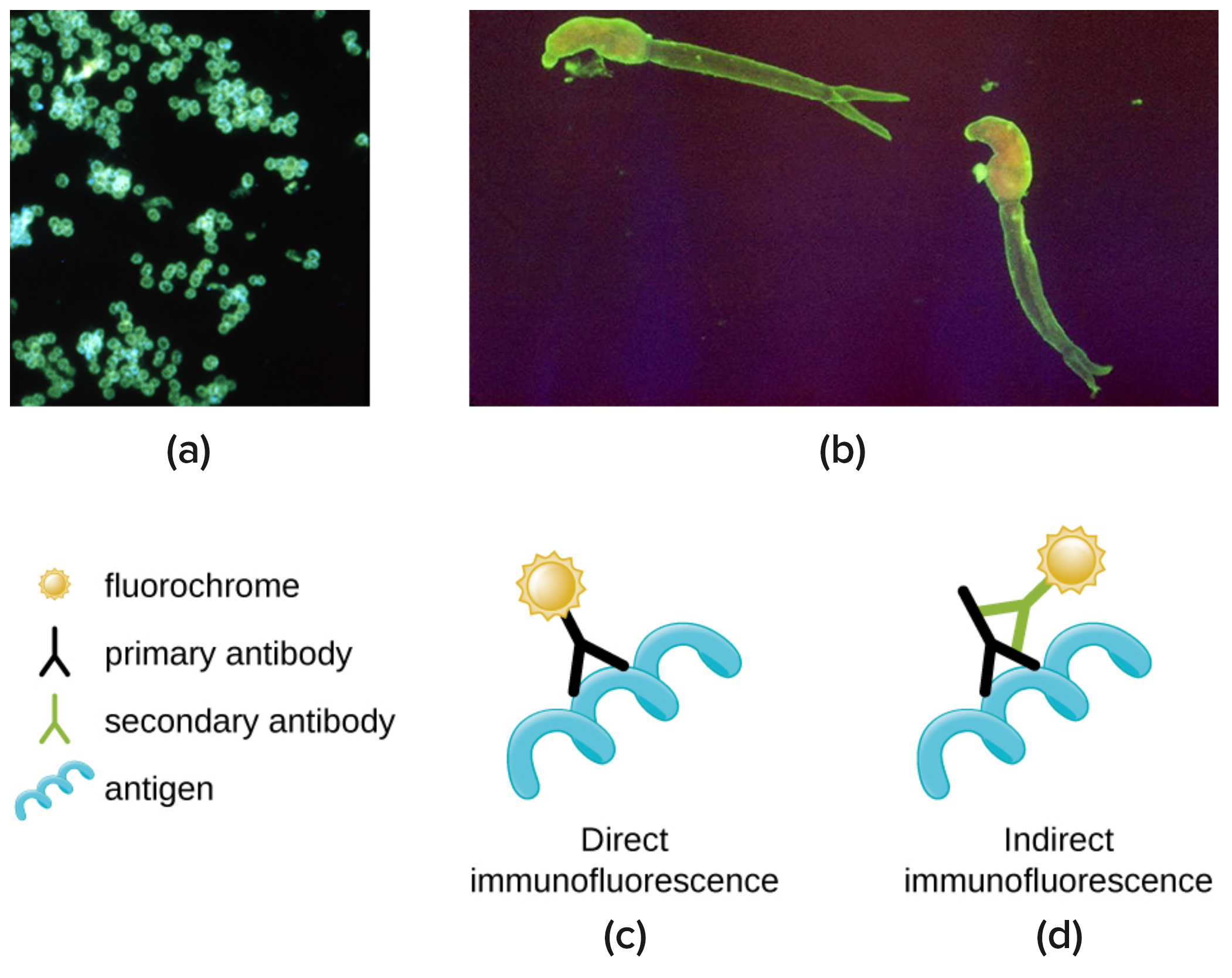
One of the most important applications of fluorescence microscopy is a technique called immunofluorescence, which is used to identify certain disease-causing microbes by observing whether antibodies bind to them. (Antibodies are protein molecules produced by the immune system that attach to specific pathogens to kill or inhibit them).In IFA, secondary antibodies rather than primary antibodies are tagged with a fluorochrome. Secondary antibodies bind to primary antibodies instead of attaching directly to the pathogen. When the unstained primary antibodies bind to the pathogen, the fluorescent secondary antibodies are attached indirectly to the pathogen. Because multiple secondary antibodies can often attach to a primary antibody, IFA increases the number of fluorescent antibodies attached to the specimen. This makes it easier to visualize features in the specimen.
Image (a) below shows a direct immunofluorescent stain used to visualize Neisseria gonorrhoeae, the bacterium that causes gonorrhea. An indirect immunofluorescent stain, shown in image (b), is used to visualize the larvae of Schistosoma mansoni, a parasitic worm that causes schistosomiasis, an intestinal disease common in the tropics. In direct immunofluorescence, shown in image (c), the stain is attache to a primary antibody, which binds to the antigen. In indirect immunofluorescence, the stain is attached by a secondary antibody, which binds to a primary antibody that has bound to the antigen.
Confocal microscopes use a laser to scan multiple z-planes successively, unlike other forms of light microscopy that create an image that is maximally focused at a single distance from the observer. This produces numerous two-dimensional, high-resolution images at various depths that can be constructed into a three-dimensional image by a computer.
Several approaches are used to improve the image. Fluorescent stains are generally used to increase contrast and resolution. Additionally, a narrow aperture eliminates any light that is not from the z-plane.
EXAMPLE
Confocal microscopes are especially useful for examining thick specimens such as this roof-dwelling cyanobacterium biofilm below.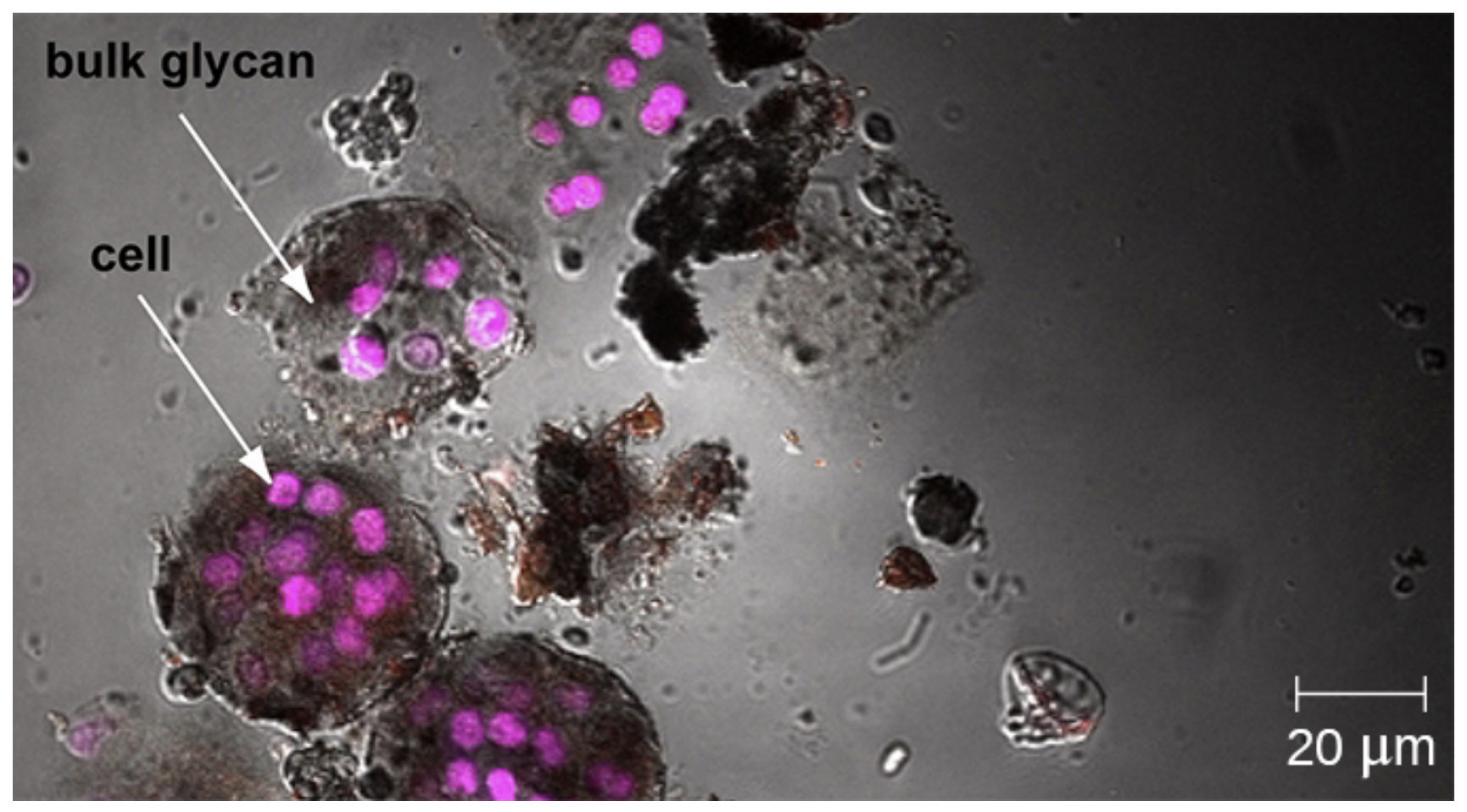
Two-photon microscopes use a scanning technique, fluorochromes, and long-wavelength light (such as infrared) to visualize specimens. The low energy associated with long-wavelength light means that two photons must strike a location at the same time to excite the fluorochrome. Two properties of the excitation light are especially important: its low-energy light is less damaging to cells than higher-energy light and its long wavelength penetrates farther than shorter-wavelength light into thick specimens.
EXAMPLE
Although the use of two-photon microscopes is limited by their high cost, they are particularly useful when available for examining living cells within intact tissues or even entire animals.| Light Microscopes | ||
|---|---|---|
| Microscope Type | Key Uses | Sample Images |
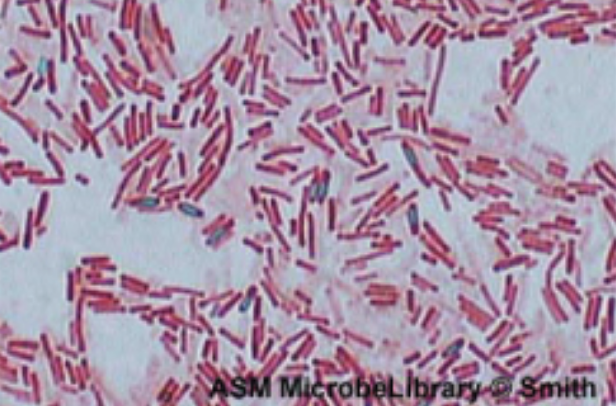
| Brightfield |
Commonly used in a wide variety of laboratory applications as the standard microscope; produces an image on a bright background.
Example: Bacillus sp. showing endospores. |
 |
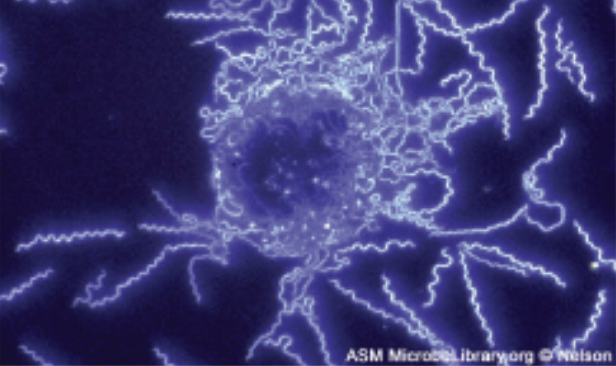
| Darkfield |
Increases contrast without staining by producing a bright image on a dark background; especially useful for viewing live specimens.
Example: Borrelia burgdorferi |
 |
| Phase Contrast |
Uses refraction and interference caused by structures in the specimen to create high-contrast, high-resolution images without staining, making it useful for viewing live specimens and structures such as endospores and organelles.
Example: Pseudomonas sp. |
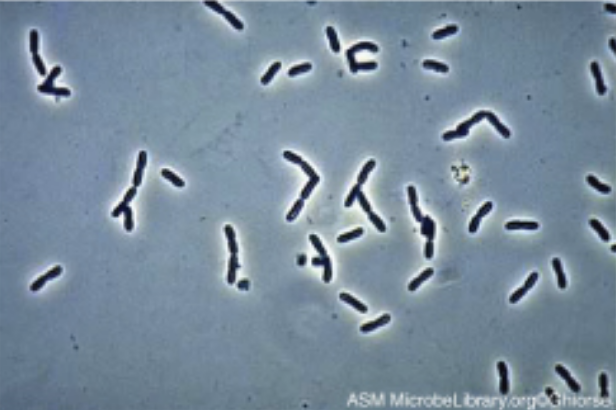 |
| Differential Interference Contrast (DIC) |
Uses interference patterns to enhance contrast between different features of a specimen to produce high-contrast images of living organisms with a three-dimensional appearance, making it especially useful in distinguishing structures within live, unstained specimens. Images viewed reveal detailed structures within cells.
Example: Escherichia coli 0157:H7 |
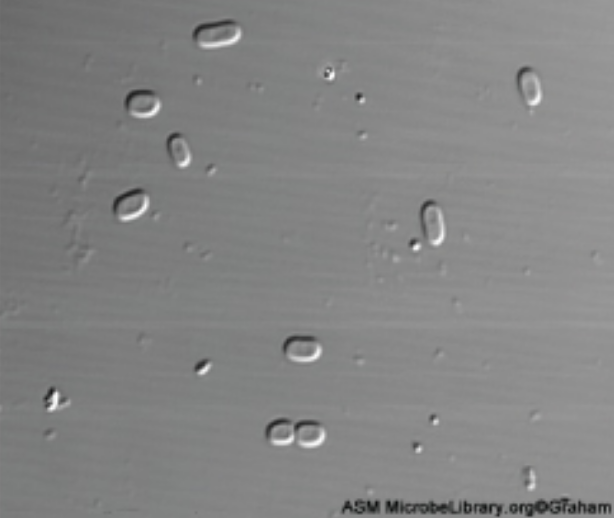 |
| Fluorescence |
Uses fluorescent stains to produce an image; can be used to identify pathogens, to find particular species, to distinguish living from dead, or to find locations of particular molecules within a cell; also used for immunofluorescence.
Example: Pseudomonas putida stained with fluorescent dyes to visualize the capsule. |
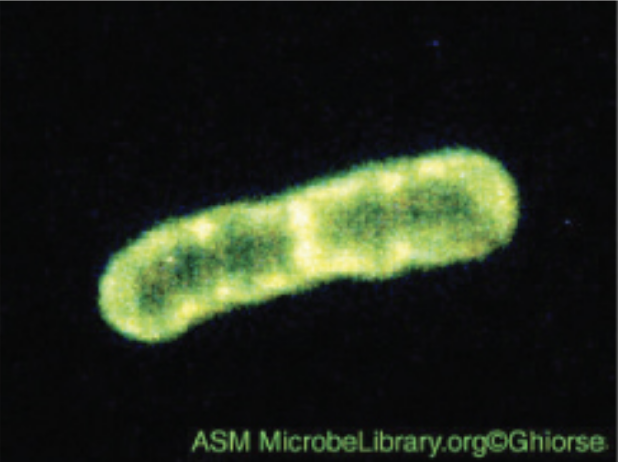 |
| Confocal |
Uses a laser to scan multiple z-planes successively, producing numerous two-dimensional, high-resolution images at various depths that can be constructed into a three-dimensional image by a computer, making this useful for examining thick specimens such as biofilms.
Example: E. coli stained with acridine orange dye to show the nucleoid regions of the cells. |
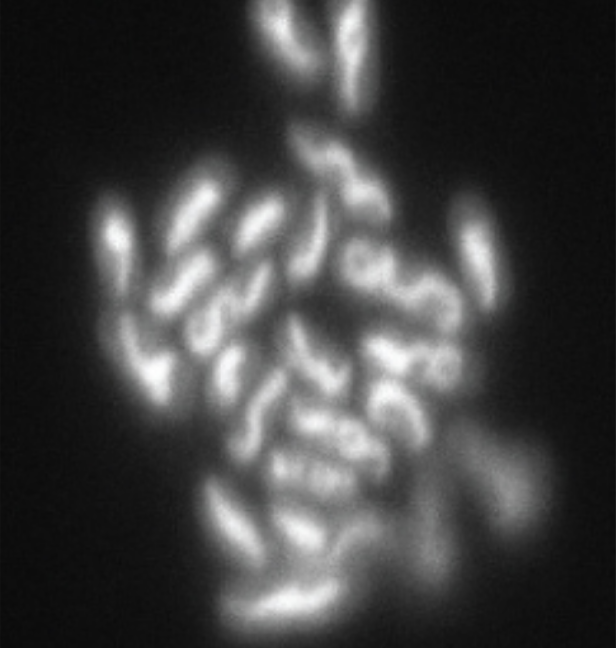 |
| Two-photon |
Uses a scanning technique, fluorochromes, and long-wavelength light (such as infrared) to penetrate deep into thick specimens such as biofilms.
Example: Mouse intestine cells stained with fluorescent dye. |
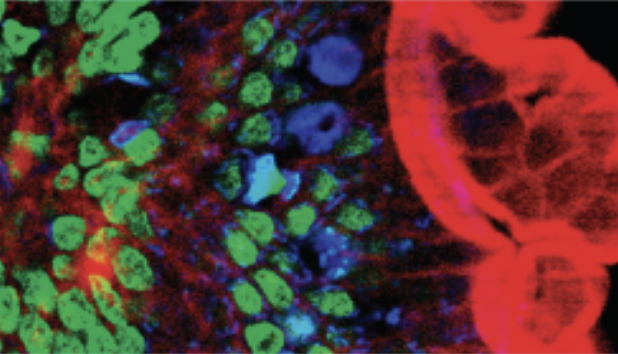 |
Electron microscopes have much higher resolution and magnification than light microscopes because the wavelengths of electron beams are so much shorter than the wavelengths of visible light.
Electron microscopes generally magnify to 100,000x and some can magnify to 2,000,000x. This allows subcellular structures and some large individual molecules to be viewed. However, living specimens cannot be viewed using electron microscopes because of the preparation involved and because a vacuum is required.
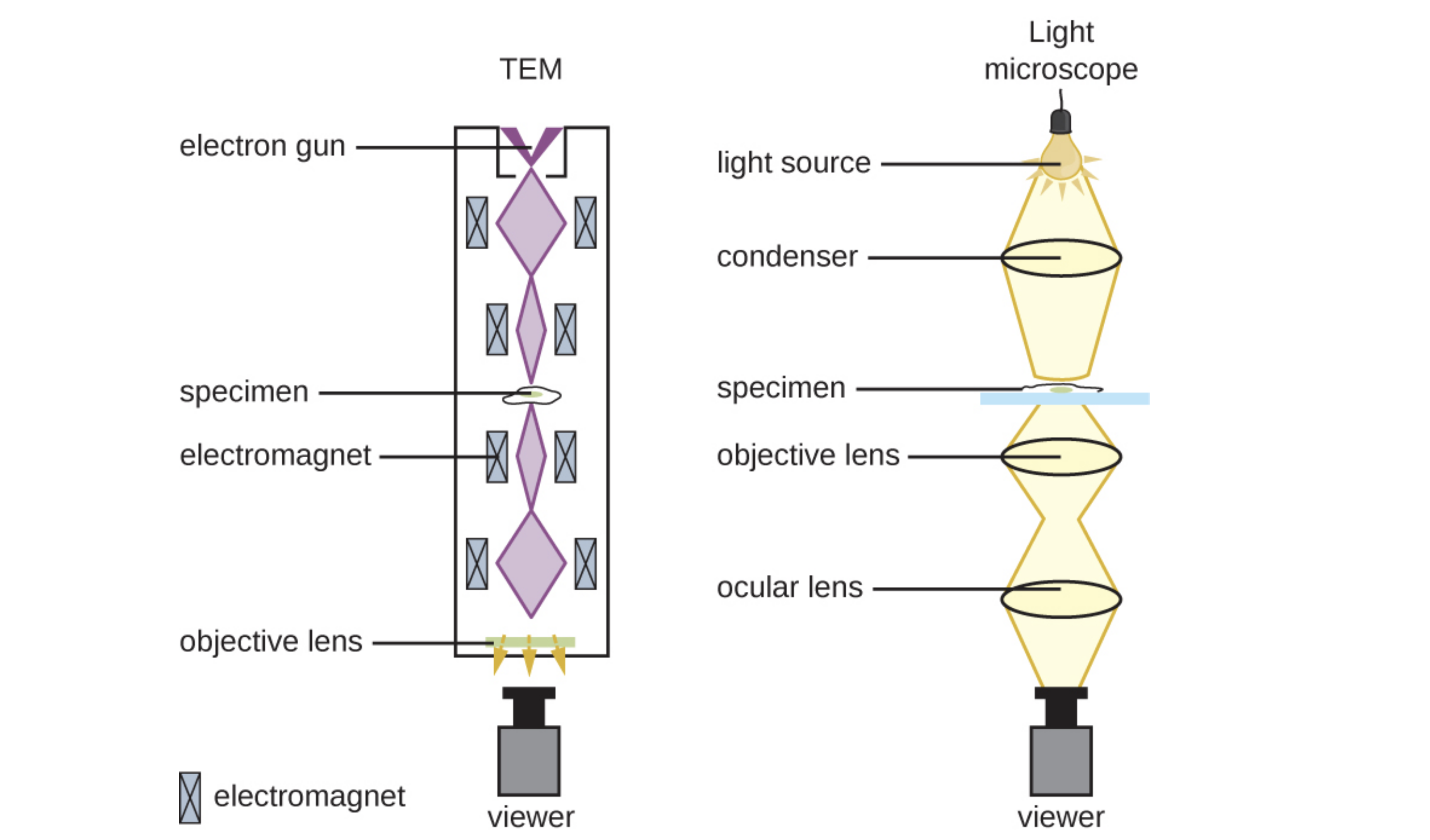
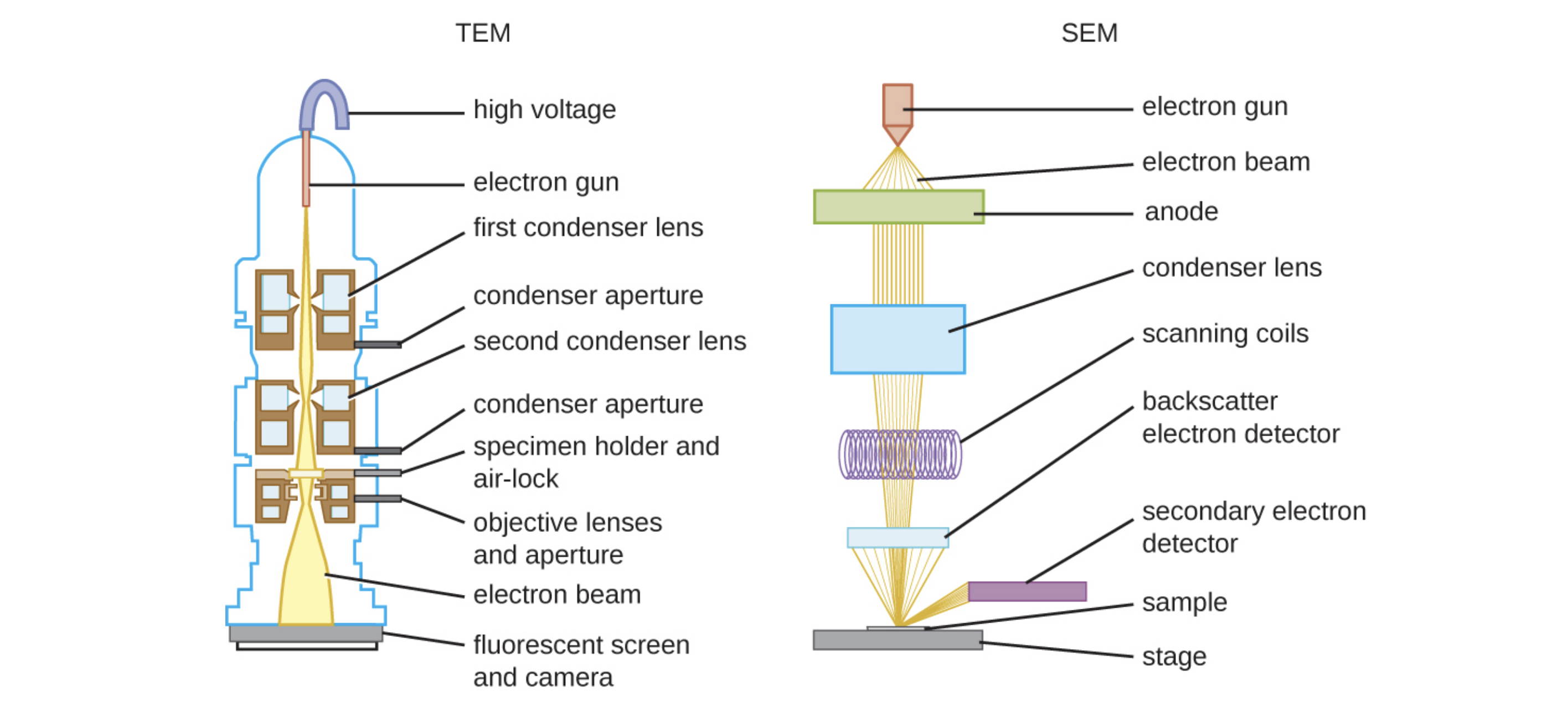
Transmission electron microscopes (TEMs) are similar to light microscopes except that they use beams of electrons instead of visible light and focus the beam using magnets instead of lenses.
Electrons pass through the specimen depending on its opacity and hit a detector that captures the image. For this to be possible, very thin (20 to 100 nm) specimens are required. Stains such as electron-dense heavy metals are used to increase opacity.
The image below shows how a TEM electron microscope uses magnets to focus electron beams similarly to the way that light microscopes use lenses to focus light.
Scanning electron microscopes (SEMs) provide high-magnification views of the three-dimensional surfaces of specimens. The specimens are dried and prepared with fixatives that reduce artifacts such as shriveling that can be produced by drying. Next, specimens are sputter-coated with a thin layer of metal such as gold. The image is formed when electrons are knocked off of specimens by an electron beam.
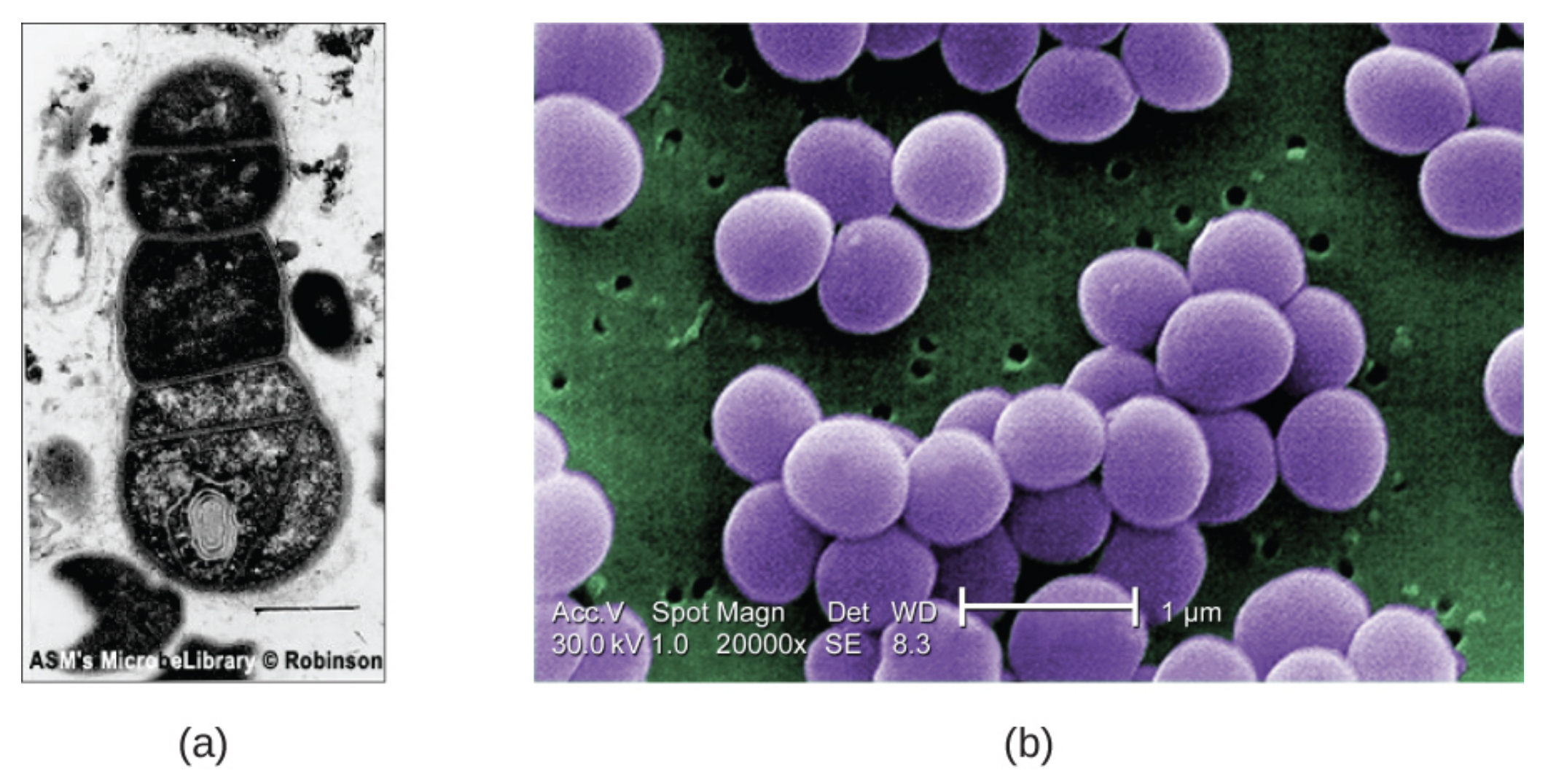
The TEM image below of cells in a biofilm (a) shows well-defined internal structures of the cells because of varying levels of opacity in the specimen. The color-enhanced SEM image (b) of the bacterium Staphylococcus aureus illustrates the ability of scanning electron microscopy to render three-dimensional images of the surface structure of cells.
| Electron Microscopes | ||
|---|---|---|
| Microscope Type | Key Uses | Sample Images |
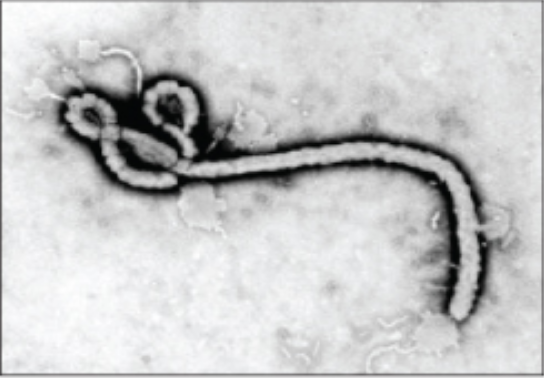
| Transmission (TEM) |
Uses electron beams that pass through a specimen to visual small images; useful to observe small, thin specimens such as tissue sections and subcellular structures.
Example: Ebola virus |
 |
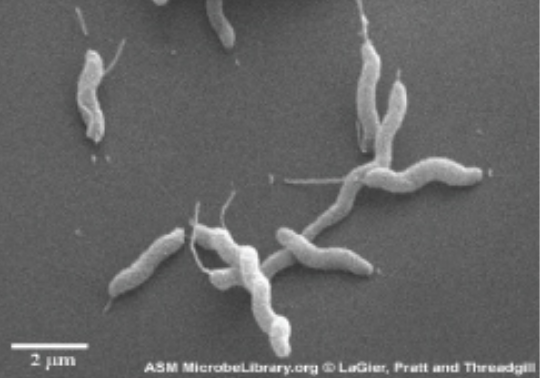
| Scanning (SEM) |
Uses electron beams to visualize surfaces; useful to observe the three-dimensional surface details of specimens.
Example: Campylobacter jejuni |
 |
| Scanning Probe Microscopes | ||
|---|---|---|
| Microscope Type | Key Uses | Sample Images |
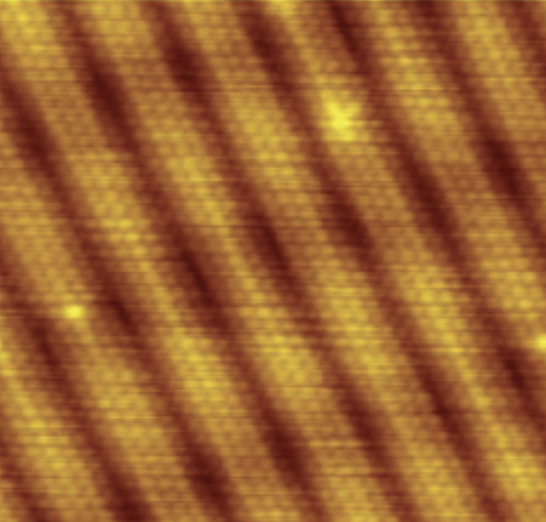
| Scanning Tunneling (STM) |
Uses a probe passed horizontally at a constant distance just above the specimen while the intensity of the current is measured; can map the structure of surfaces at the atomic level; works best on conducting materials but can also be used to examine organic materials such as DNA if fixed on a surface.
Example: Image of surface reconstruction on a clean gold [Au(100)] surface, as visualized using scanning tunneling microscopy. |
 |
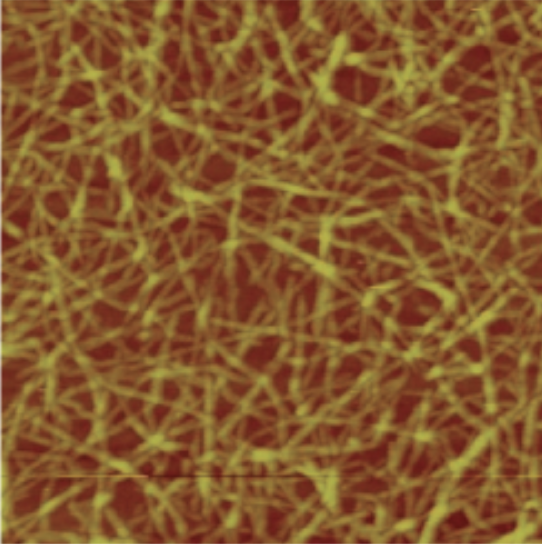
| Atomic Force (AFM) |
Can be used in several ways, including using a laser focused on a cantilever to measure the bending of the tip or a probe passed above the specimen while the height needs to maintain a constant current is measured; useful to observe specimens at the atomic level and can be more easily used with nonconducting samples.
Example: AFM height image of carboxymethylated nanocellulose adsorbed on a silica surface. |
 |
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “MICROBIOLOGY.” ACCESS FOR FREE AT openstax.org/details/books/microbiology. LICENSE: CC ATTRIBUTION 4.0 INTERNATIONAL.
REFERENCES
Parker, N., Schneegurt, M., Thi Tu, A.-H., Lister, P., & Forster, B. (2016). Microbiology. OpenStax.
Access for free at openstax.org/books/microbiology/pages/1-introduction