Table of Contents |
With up to 180 liters per day passing through the nephrons of the kidney, it is quite obvious that most of that fluid and its contents must be reabsorbed. That recovery occurs in the proximal convoluted tubule (PCT), loop of Henle, distal convoluted tubule (DCT), and collecting ducts (see the areas in the image below). Various portions of the nephron differ in their capacity to reabsorb water and specific solutes.
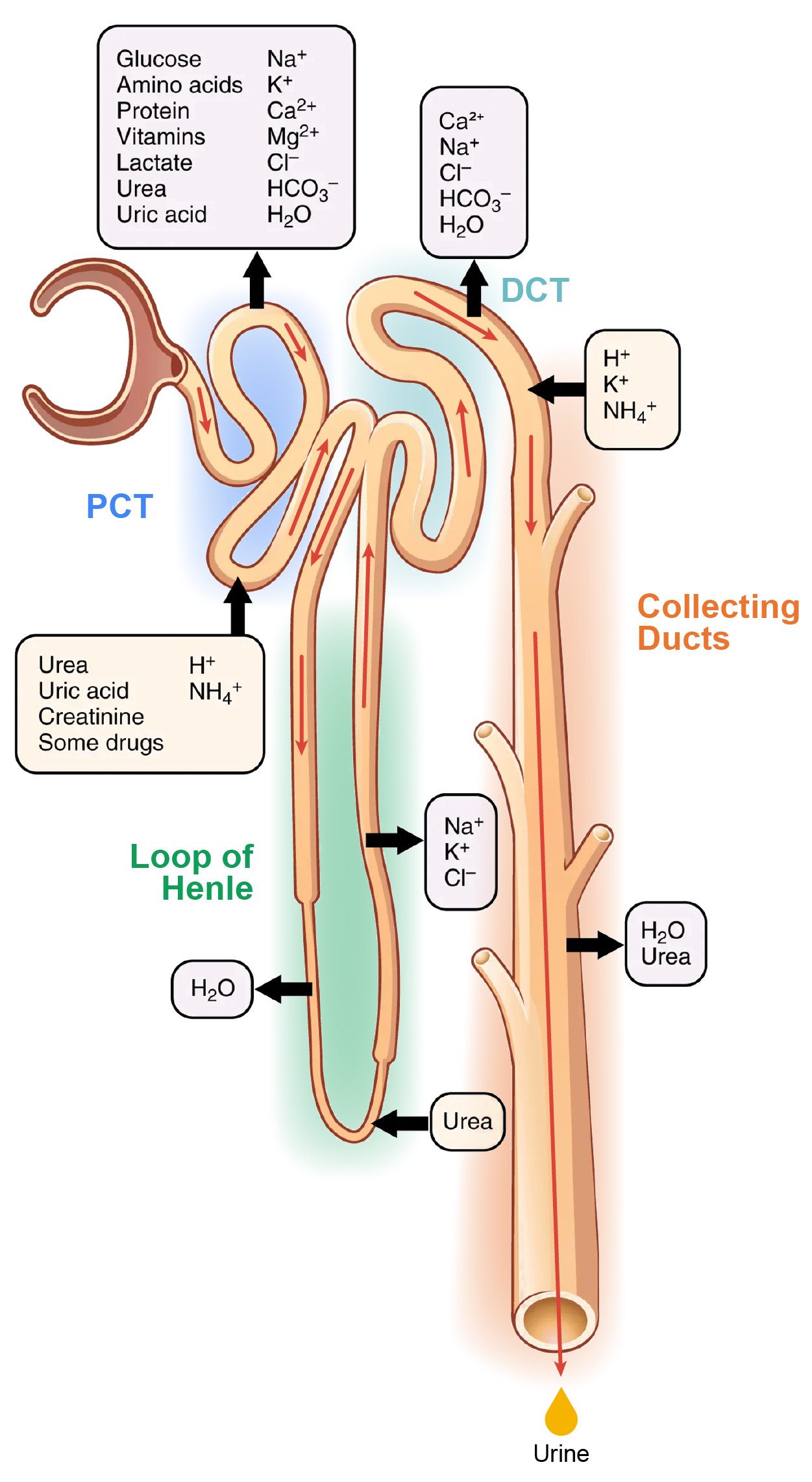
While much of the reabsorption and secretion occur passively based on concentration gradients, the amount of water that is reabsorbed or lost is tightly regulated. This control is exerted directly by antidiuretic hormone (ADH) and aldosterone, and indirectly by renin. Recall that in a previous lesson, you learned that ADH is a hormone secreted by the hypothalamus that signals water reabsorption by the kidneys. Most water is recovered in the PCT, loop of Henle, and DCT. About 10% (about 18 L) reaches the collecting ducts. The collecting ducts, under the influence of ADH, can recover almost all of the water passing through them, in cases of dehydration, or almost none of the water, in cases of over-hydration.
Mechanisms by which substances move across membranes for reabsorption or secretion include active transport, diffusion, facilitated diffusion, secondary active transport, and osmosis.
Active transport utilizes energy, usually the energy found in a phosphate bond of ATP, to move a substance across a membrane from a low to a high concentration. It is very specific and must have an appropriately shaped receptor for the substance to be transported. The transport of Na⁺ out of a cell and K⁺ into a cell by the Na⁺/K⁺ pump is an example of active transport. Both ions are moved in opposite directions from a lower to a higher concentration.
Simple diffusion moves a substance from a higher to a lower concentration down its concentration gradient. It requires no energy and only needs to be soluble.
Facilitated diffusion is similar to diffusion in that it moves a substance down its concentration gradient. The difference is that it requires specific membrane receptors or channel proteins for movement. The movement of glucose and, in certain situations, Na⁺ ions is an example of facilitated diffusion. In some cases of mediated transport, two different substances share the same channel protein port; these mechanisms are described by the terms symport and antiport.
Symport mechanisms move two or more substances in the same direction at the same time, whereas antiport mechanisms move two or more substances in opposite directions across the cell membrane. Both mechanisms may utilize concentration gradients maintained by ATP pumps. As described previously, when active transport powers the transport of another substance in this way, it is called “secondary active transport.” Glucose reabsorption in the kidneys is by secondary active transport. Na⁺/K⁺ ATPases on the basal membrane of a tubular cell constantly pump Na⁺ out of the cell, maintaining a strong electrochemical gradient for Na⁺ to move into the cell from the tubular lumen. On the luminal (apical) surface, a Na⁺/glucose symport protein assists both Na⁺ and glucose movement into the cell. The cotransporter moves glucose into the cell against its concentration gradient as Na⁺ moves down the electrochemical gradient created by the basal membranes Na⁺/K⁺ ATPases. The glucose molecule then diffuses across the basal membrane by facilitated diffusion into the interstitial space and from there into peritubular capillaries.
Most of the Ca²⁺, Na⁺, glucose, and amino acids must be reabsorbed by the nephron to maintain homeostatic plasma concentrations. Other substances, such as urea, K⁺, ammonia (NH₃), creatinine, and some drugs are secreted into the filtrate as waste products. Acid–base balance is maintained through actions of the lungs and kidneys: the lungs rid the body of H⁺, whereas the kidneys secrete or reabsorb H⁺ and HCO₃⁻ . In the case of urea, about 50% is passively reabsorbed by the PCT. More is recovered by the collecting ducts as needed. ADH induces the insertion of urea transporters and aquaporin channel proteins.
The renal corpuscle filters the blood to create a filtrate that differs from blood mainly in the absence of cells and large proteins. From this point to the ends of the collecting ducts, the filtrate undergoes modification through secretion and reabsorption before true urine is produced.
The first point at which the forming urine is modified is in the PCT. Here, some substances are reabsorbed, whereas others are secreted. Note the use of the term “reabsorbed.” All of these substances were “absorbed” in the digestive tract—99% of the water and most of the solutes filtered by the nephron must be reabsorbed. Water and substances that are reabsorbed are returned to the circulation by the peritubular and vasa recta capillaries.

It is important to understand the difference between the glomerulus and the peritubular and vasa recta capillaries. The glomerulus has a relatively high pressure inside its capillaries and can sustain this by dilating the afferent arteriole while constricting the efferent arteriole. This assures adequate filtration pressure even as the systemic blood pressure varies. The movement of water into the peritubular capillaries and vasa recta will be primarily influenced by osmolarity and concentration gradients.
The movement of many solutes and water from the PCT lumen into the interstitial spaces between cells occurs through two main pathways:
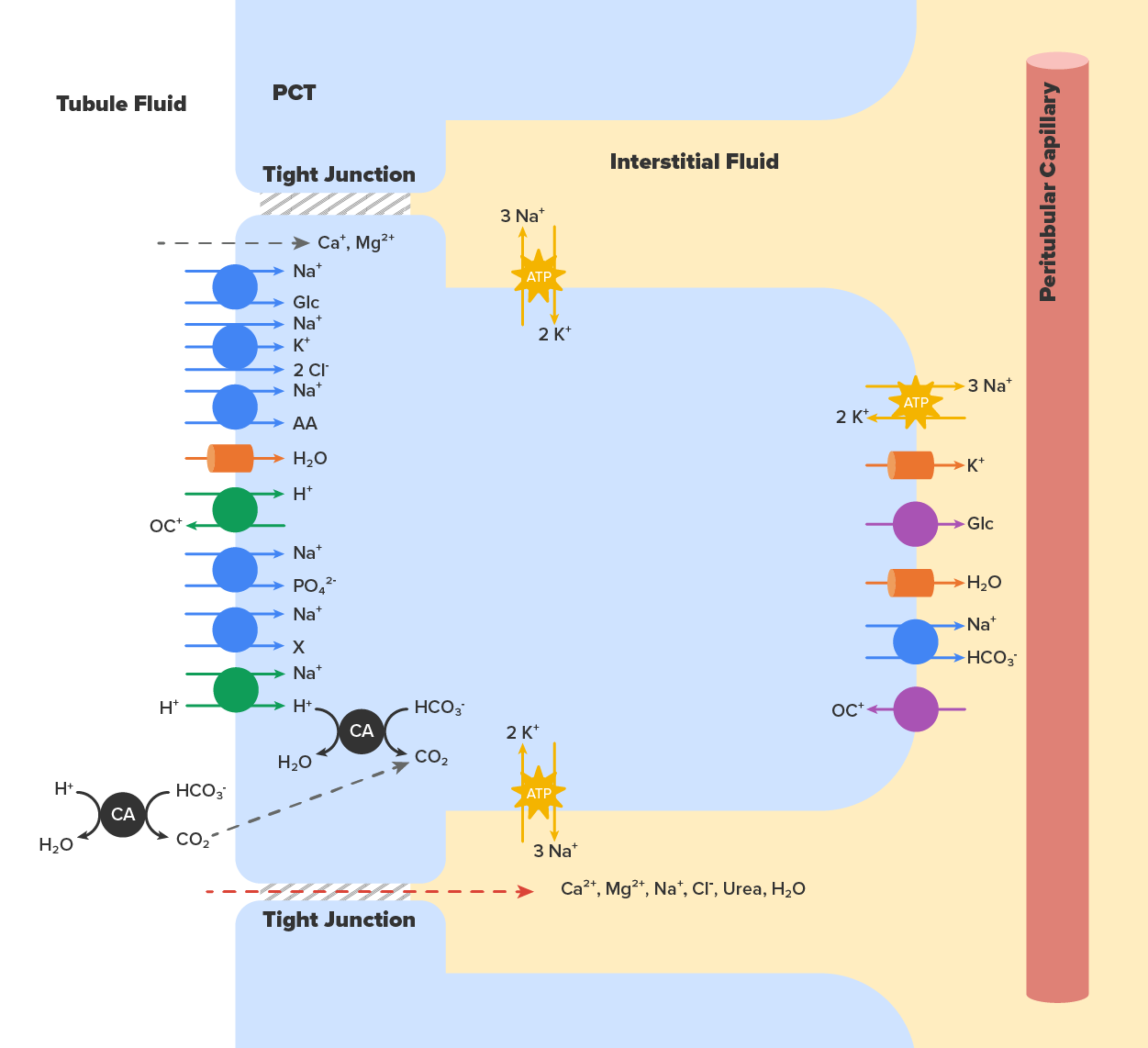
In the PCT, approximately ⅔ of the filtered Na⁺, K⁺, and H₂O are reabsorbed. All of the filtered glucose is reabsorbed. Most bicarbonate (80–90%) is reabsorbed. Phosphate (85%) is reabsorbed, but inhibited by parathyroid hormone (PTH).
The loop of Henle consists of two sections: thick and thin descending and thin and thick ascending sections. The loops of cortical nephrons do not extend into the renal medulla very far, if at all. Juxtamedullary nephrons have loops that extend variable distances, some very deep into the medulla. The descending and ascending portions of the loop are highly specialized to enable the recovery of much of the Na⁺ and water that was filtered by the glomerulus.
As the forming urine moves through the loop, the osmolarity will change from isosmotic with blood (about 278–300 mOsmol/kg) to both a very hypertonic solution of about 1200 mOsmol/kg and a very hypotonic solution of about 100 mOsmol/kg. These changes are accomplished by osmosis in the descending limb and active transport in the ascending limb. Solutes and water recovered from these loops are returned to the circulation by way of the vasa recta.
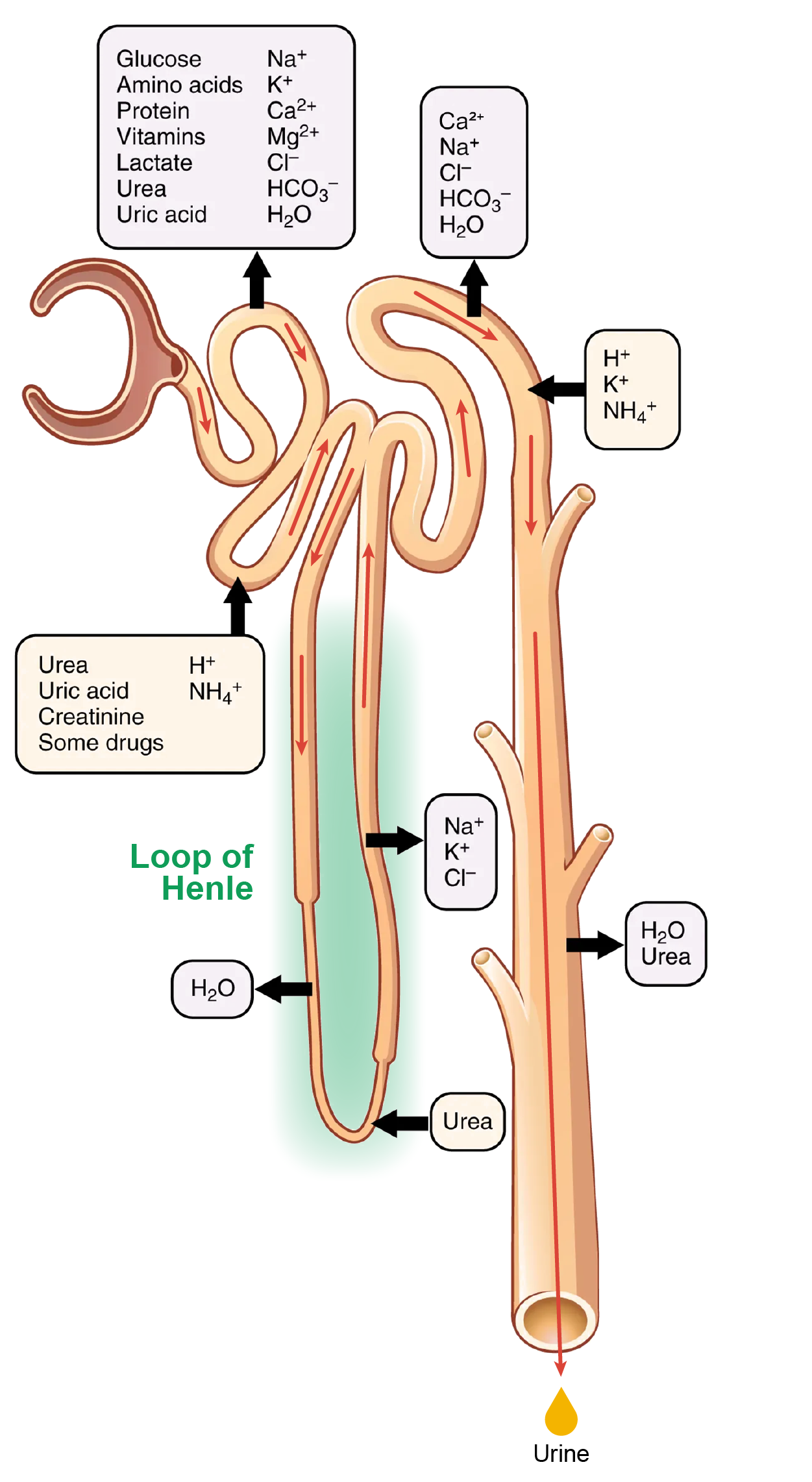
What is happening in the Loop of Henle:
The majority of the descending loop consists of simple squamous epithelial cells; to simplify the function of the loop, this discussion focuses on these cells. These membranes have permanent aquaporin channel proteins that allow unrestricted movement of water from the descending loop into the surrounding interstitium as osmolarity increases from about 300 mOsmol/kg to about 1200 mOsmol/kg in the filtrate. This increase results in the reabsorption of up to 15% of the water entering the nephron. Modest amounts of urea, Na⁺, and other ions are also recovered here.
Most of the solutes that were filtered in the glomerulus have now been recovered along with the majority of water, about 82%. As the forming urine enters the ascending loop, major adjustments will be made to the concentration of solutes to create what you perceive as urine.
The ascending loop is made of very short thin and longer thick portions. Once again, to simplify the function, this section only considers the thick portion. The thick portion is lined with simple cuboidal epithelium without a brush border. It is completely impermeable to water due to the absence of aquaporin proteins, but ions, mainly Na⁺ and Cl⁻, are actively reabsorbed by a cotransport system. This has two significant effects: removal of NaCl while retaining water leads to a hypoosmotic filtrate by the time it reaches the DCT; pumping NaCl into the interstitial space contributes to the hyperosmotic environment in the kidney medulla.
The Na⁺/K⁺ ATPase pumps in the basal membrane create an electrochemical gradient, allowing reabsorption of Cl⁻ by Na⁺/Cl⁻ symporters in the apical membrane. At the same time that Na⁺ is actively pumped from the basal side of the cell into the interstitial fluid, Cl⁻ follows the Na⁺ from the lumen into the interstitial fluid by a paracellular route by solvent drag.
Most of the K⁺ that enters the cell via symporters returns to the lumen (down its concentration gradient) through leaky channels in the apical membrane. Note the environment now created in the interstitial space: with the “back door exiting” K⁺, there is one Na⁺ and two Cl⁻ ions left in the interstitium surrounding the ascending loop. Therefore, compared with the lumen of the loop, the interstitial space is now a negatively charged environment. This negative charge attracts cations (Na⁺, K⁺, Ca²⁺, and Mg²⁺) from the lumen via a paracellular route to the interstitial space and vasa recta.
The structure of the loop of Henle and associated vasa recta create a countercurrent multiplier system. The countercurrent term comes from the fact that the descending and ascending loops are next to each other and their fluid flows in opposite directions (countercurrent). The multiplier term is because of the action of solute pumps that increase (multiply) the concentrations of urea and Na⁺ deep in the medulla.
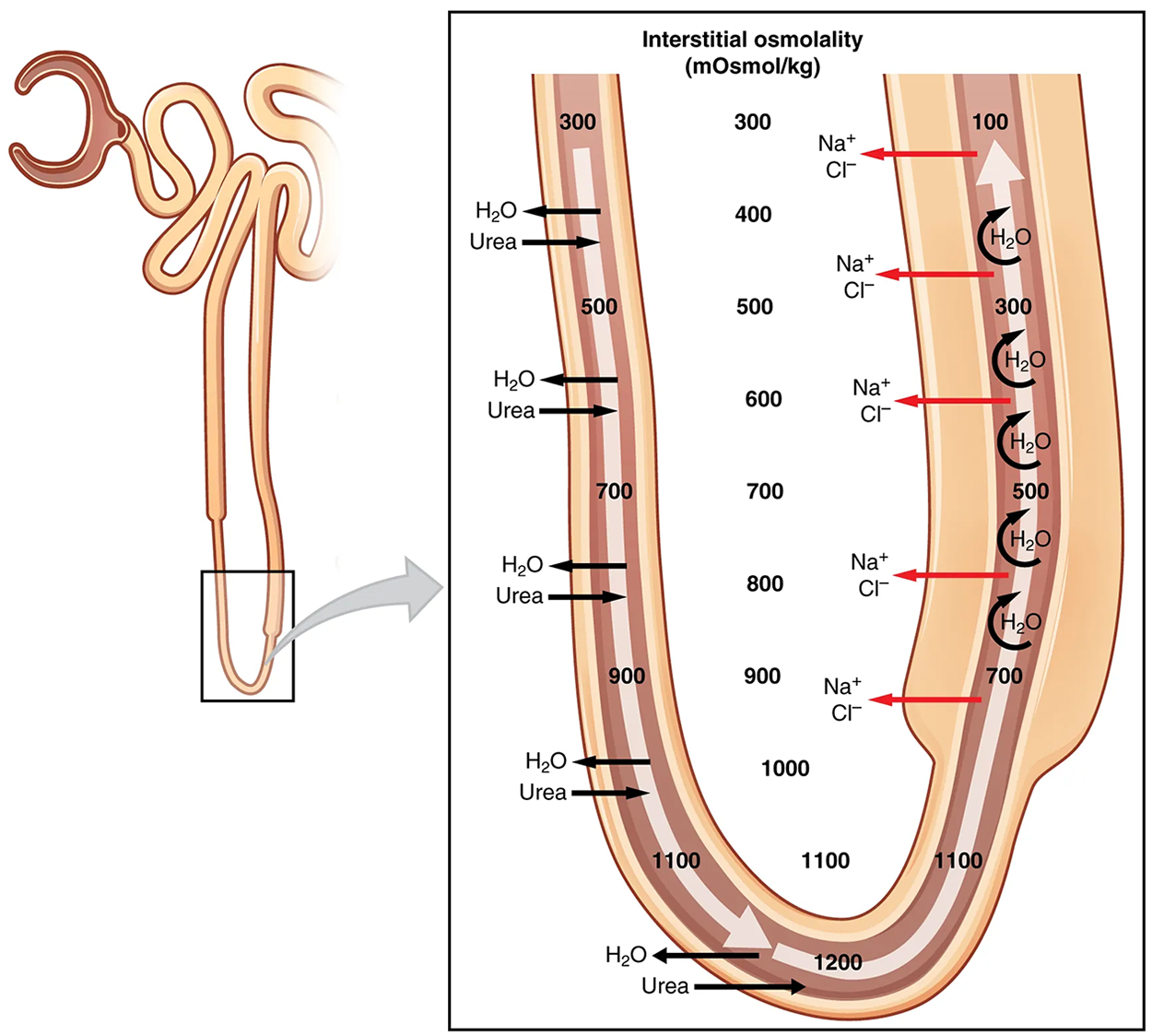
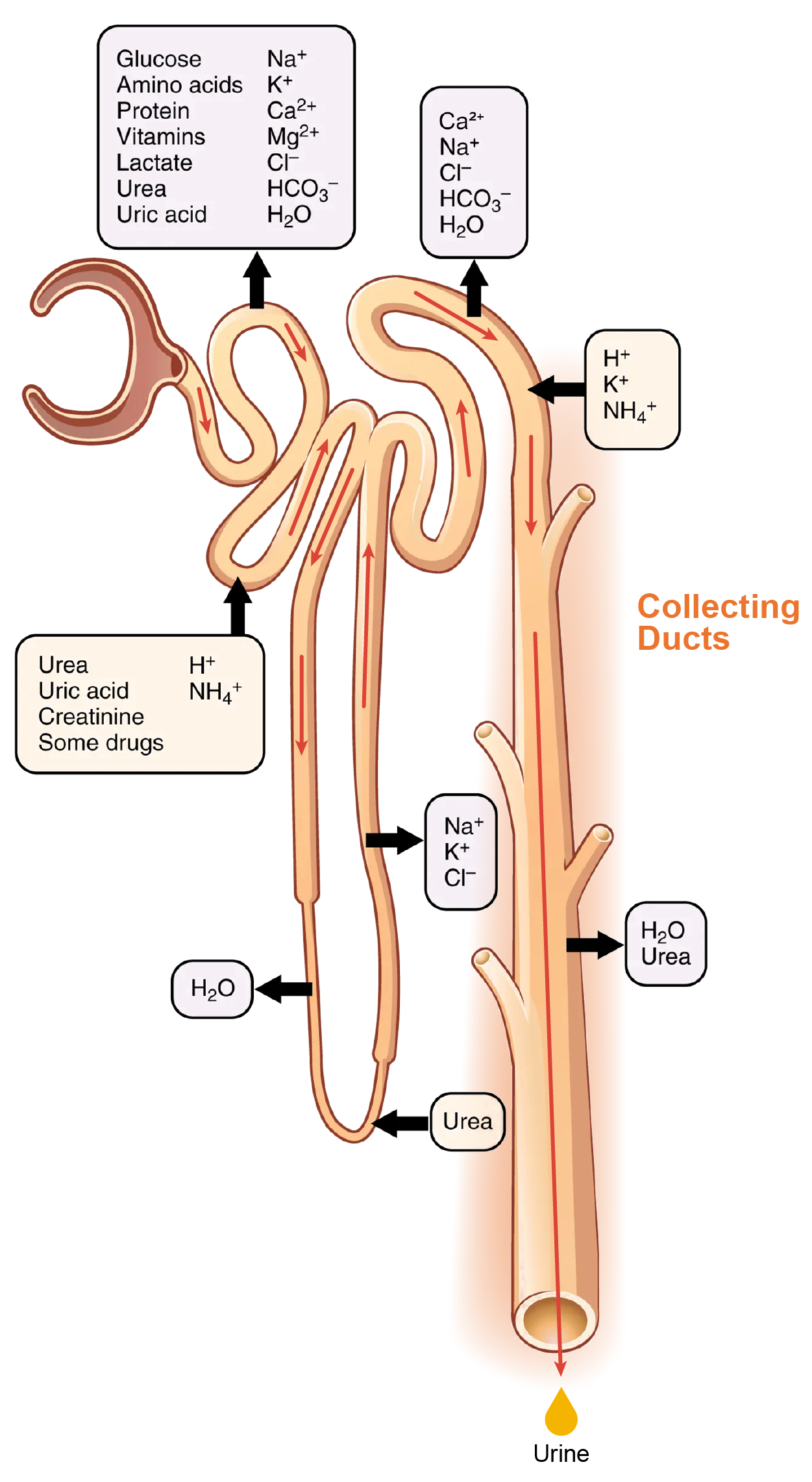
As discussed above, the ascending loop actively reabsorbs NaCl out of the forming urine into the interstitial spaces. In addition, collecting ducts have urea pumps that actively pump urea into the interstitial spaces. This results in the recovery of NaCl to the circulation via the vasa recta and creates a high osmolar environment in the depths of the medulla.
At the same time that water is freely diffusing out of the descending loop through aquaporin channels into the interstitial spaces of the medulla, urea freely diffuses into the lumen of the descending loop as it descends deeper into the medulla, much of it to be reabsorbed from the forming urine when it reaches the collecting duct. Thus, the movement of Na⁺ and urea into the interstitial spaces by these mechanisms creates the hyperosmotic environment of the medulla. The countercurrent multiplier system of the loop of Henle sets up an osmotic gradient from low in superficial portions to high in deep portions of the medulla. This gradient is important in recovering both water from the collecting ducts as you will see below.
As the loop turns to become the ascending loop, there is an absence of aquaporin channels, so water cannot leave the loop. However, in the basal membrane of cells of the thick ascending loop, ATPase pumps actively remove Na+ from the cell. A Na⁺/K⁺/2Cl⁻ symporter in the apical membrane passively allows these ions to enter the cell cytoplasm from the lumen of the loop down a concentration gradient created by the pump. This mechanism works to dilute the fluid of the ascending loop ultimately to approximately 50–100 mOsmol/L.
At the transition from the DCT to the collecting duct, about 20% of the original water is still present and about 10% of the sodium. If no other mechanism for water reabsorption existed, about 20–25 liters of urine would be produced. Now consider what is happening in the adjacent capillaries, the vasa recta. They are recovering both solutes and water at a rate that preserves the countercurrent multiplier system.
In general, blood flows slowly in capillaries to allow time for the exchange of nutrients and wastes. In the vasa recta particularly, this rate of flow is important for two additional reasons. First, the flow must be slow to allow blood cells to lose and regain water without either crenating or bursting. Second, a rapid flow would remove too much Na⁺ and urea, destroying the osmolar gradient that is necessary for the recovery of solutes and water. Thus, by flowing slowly to preserve the countercurrent mechanism, as the vasa recta descend, Na⁺ and urea are freely able to enter the capillary, while water freely leaves; as they ascend, Na⁺ and urea are secreted into the surrounding medulla, while water reenters and is removed.
Approximately 80% of filtered water has been recovered by the time the dilute forming urine enters the DCT.
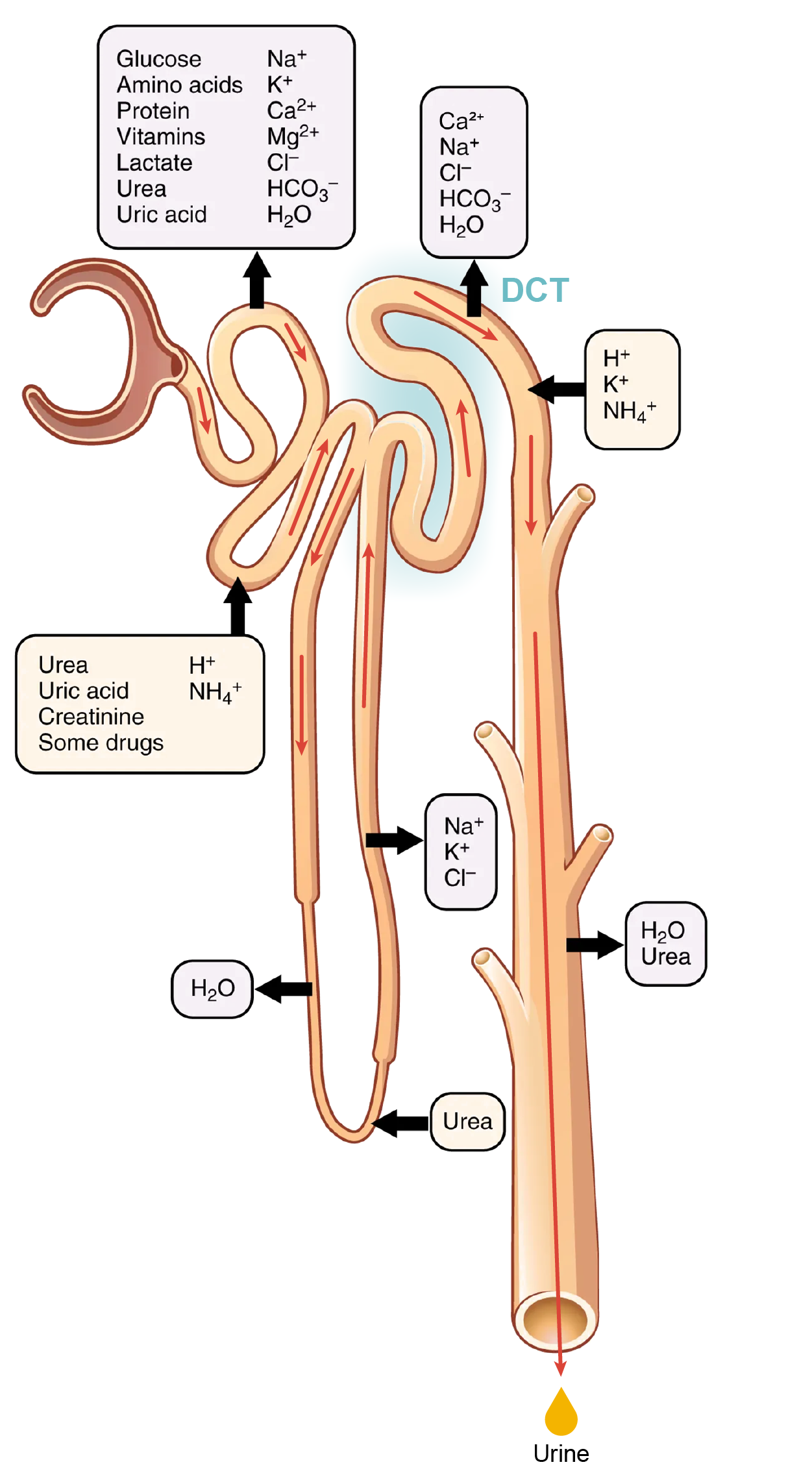
What is happening in the early DCT:
Solutes move across the membranes of the collecting ducts, which contain two distinct cell types, principal cells and intercalated cells. A principal cell possesses channels for the recovery or loss of sodium and potassium. An intercalated cell secretes or absorbs acid or bicarbonate. As in other portions of the nephron, there is an array of micromachines (pumps and channels) on display in the membranes of these cells.
What is happening in late DCT and collecting ducts:
This function is regulated by the posterior pituitary hormone ADH (vasopressin). With mild dehydration, plasma osmolarity rises slightly. This increase is detected by osmoreceptors in the hypothalamus, which stimulates the release of ADH from the posterior pituitary. If plasma osmolarity decreases slightly, the opposite occurs.
When stimulated by ADH, aquaporin channels are inserted into the apical membrane of principal cells, which line the collecting ducts. As the ducts descend through the medulla, the osmolarity surrounding them increases (due to the countercurrent mechanisms described above). If aquaporin water channels are present, water will be osmotically pulled from the collecting duct into the surrounding interstitial space and into the peritubular capillaries. Therefore, the final urine will be more concentrated.
If less ADH is secreted, fewer aquaporin channels are inserted and less water is recovered, resulting in dilute urine. By altering the number of aquaporin channels, the volume of water recovered or lost is altered. This, in turn, regulates the blood osmolarity, blood pressure, and osmolarity of the urine.
As Na⁺ is pumped from the forming urine, water is passively recaptured for circulation; this preservation of vascular volume is critically important for the maintenance of normal blood pressure. Aldosterone is secreted by the adrenal cortex in response to angiotensin II stimulation. Recall that you previously learned that angiotensin II is an extremely potent vasoconstrictor that functions immediately to increase blood pressure. By also stimulating aldosterone production, it provides a longer-lasting mechanism to support blood pressure by maintaining vascular volume (water recovery).
In addition to receptors for ADH, principal cells have receptors for the steroid hormone aldosterone. While ADH is primarily involved in the regulation of water recovery, aldosterone regulates Na⁺ recovery. Aldosterone stimulates principal cells to manufacture luminal Na⁺ and K⁺ channels as well as Na⁺/K⁺ ATPase pumps on the basal membrane of the cells.
Symport channels move Na⁺ and Cl⁻ together. Still, other channels in the principal cells secrete K⁺ into the collecting duct in direct proportion to the recovery of Na⁺.
Intercalated cells also play significant roles in regulating blood pH. Intercalated cells reabsorb K⁺ and HCO₃⁻ while secreting H⁺. This function lowers the acidity of the plasma while increasing the acidity of the urine.
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX "ANATOMY AND PHYSIOLOGY 2E" ACCESS FOR FREE AT OPENSTAX.ORG/DETAILS/BOOKS/ANATOMY-AND-PHYSIOLOGY-2E. LICENSE: CREATIVE COMMONS ATTRIBUTION 4.0 INTERNATIONAL