Table of Contents |
In early lessons in this course, you learned about some of the earliest examples of humans attempting to treat infections. For example, you have learned about the ways in which people have tried to preserve food by dehydrating, candying, and fermenting it. You also learned about the discovery of a 5,300-yr-old frozen mummy discovered in 1991 that may have been trying to use a medicinal fungus to treat an infection.
There is abundant evidence of the ways in which people have tried to use chemicals to treat diseases. For example, people in Nubia (a place between Sudan and Egypt) may have used tetracycline produced during beer-making to treat ailments between 350 and 550 AD. Fungi and herbs such as Bupleurum Root, Asian Ginseng Root, Astragalus Root, and Chinese Hawthorn Berry, have been used for centuries, and recent research has identified antimicrobial properties in some of these traditional medicines.
The image below shows examples of plants that have been used by Chinese herbalists for millennia.

Beginning in the first half of the 20th century, scientists began to systematically research antimicrobial drugs. This involved specifically screening compounds to determine which were effective against particular microbes.
 Sahachiro Hata (1873–1938)
Sahachiro Hata (1873–1938) 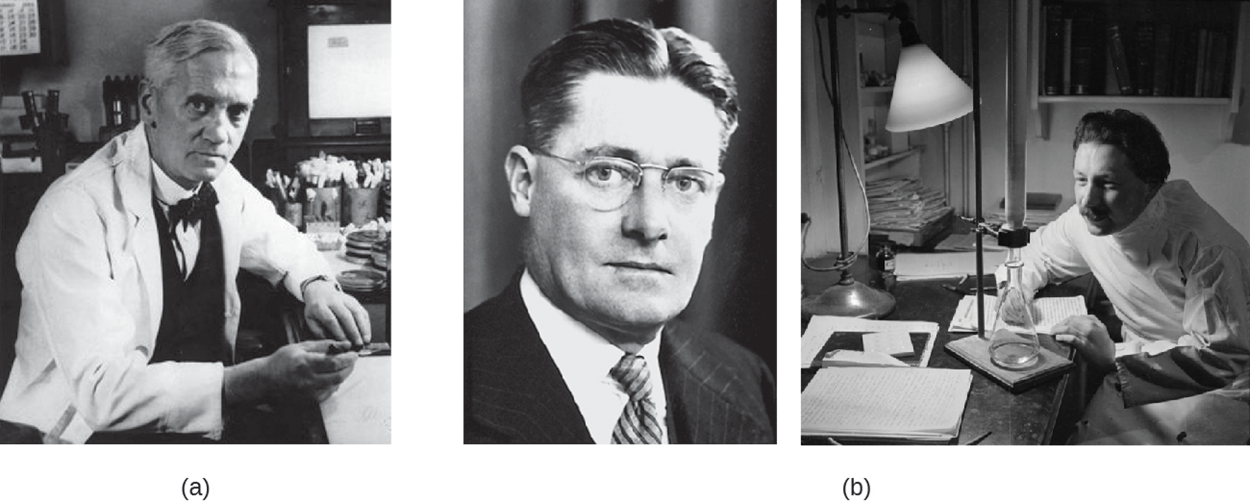
Although Fleming and his colleagues discovered penicillin, it took additional work by other researchers to purify penicillin, develop ways to mass produce it for widespread use, and test its effectiveness in humans. The team that accomplished this research was led by Howard Florey (1898–1968) seen in the left photo (b) above and Ernst Chain (1906–1979) seen in the right photo (b) above. As a result of these accomplishments, Fleming, Florey, and Chain were awarded the Nobel Prize in Physiology and Medicine in 1945.
Although its antimicrobial properties were discovered after penicillin, a group of German scientists discovered that a synthetic dye (prontosil) could treat streptococcal and staphylococcal infections in mice. One of these researchers, Gerhard Domagk (1895–1964), received the Nobel Prize in Physiology or Medicine in 1939 for his work with both prontosil and the broken down product of prontosil in the body, sulfanilamide. Because it was developed chemically rather than found in nature, sulfanilamide is a synthetic antimicrobial. Additionally, the identification of the antimicrobial properties of sulfanilamide led to the development of related sulfa drugs and later to the discovery of other synthetic antimicrobials such as quinolines and oxazolidinones.
By using x-rays to analyze the structures of chemicals, scientist Dorothy Hodgkin determined the structure of penicillin in 1946 and received the Nobel Prize in Chemistry in 1964. This detailed understanding of the structure of penicillin allowed scientists to create modified versions called semisynthetic antimicrobials (artificially modified versions of chemicals found in nature). This meant that scientists could make modifications to improve medications in a variety of ways. For example, they could improve stability, decrease toxicity, and increase the range of bacteria targeted.
In the 1940s, soil microbiologist Selman Waksman (1888–1973) led a research team studying actinomycete fungi that discovered a variety of antimicrobials (actinomycin, streptomycin, and neomycin). Over half of all natural antibiotics are produced by actinomycetes. For his work, Waksman received the Nobel Prize in Physiology or Medicine in 1952.
In this lesson, you first learned about the early ways in which people attempted to use medications to treat infections and then how approaches to drug development became increasingly sophisticated. The effort to find effective new medications continues. As new diseases appear and pathogens develop resistance that allows them to survive existing treatments, researchers need to find new versions of existing medications and try to identify entirely new classes of compounds that may be useful.
As you learn about antimicrobial drugs, you will learn more and more about why antimicrobial resistance is a major concern. Whenever antimicrobials are used, there is a risk of the development of resistant microbes. This means that the microbes are no longer inhibited or killed by the medication, making it less useful. Selection and resistance occur in all types of microbes, and you will learn specifically about resistance in bacteria in another lesson.
EXAMPLE
Staphylococcus aureus is a very common bacterium often found on human skin and associated with infections. In the community, it generally causes skin infections. However, it can also cause other types of infections and even lethal, systemic infections (meaning infections that spread through the body). Resistant strains of S. aureus are becoming increasingly problematic. Methicillin-resistant S. aureus (MRSA) is more difficult to treat than nonresistant S. aureus because it is resistant to several antibiotics (including methicillin). MRSA is common in the community and in health care settings, so health care professionals now consider it a likely cause when they encounter patients with infections that meet its profile (CDC, 2019). Between 2002 and January 2022, the Centers for Disease Control (CDC) documented 16 cases of an even more resistant strain of MRSA called vancomycin-resistant S. aureus (VRSA) and warns that resistance genes can be transferred from vancomycin-resistant enterococci (VRE) to MRSA (CDC, 2022). This illustrates how the emergence of increasingly resistant strains of S. aureus and other bacterial species is a major public health concern.There are ways to lower the risk of resistance. For example, individuals with HIV are generally prescribed cocktails of multiple medications because it is less likely for a virus to develop multiple different types of resistance simultaneously than to develop resistance to a single medication.
Additionally, careful prescription approaches that avoid the overuse of antibiotics and encourage patients to take antibiotics as prescribed can help to reduce resistance. Whenever antibiotics are used, the risk of resistance is increased because the most resistant bacteria survive antibiotic treatment. If someone is given an antibiotic and takes it briefly, the antibiotic will kill the most susceptible bacteria, but more resistant ones may survive. If antibiotic treatment is stopped too quickly, the most resistant bacteria are left and can spread. By reserving antibiotic use for times that it is necessary and encouraging patients to take the full recommended amount, the opportunities for resistant bacteria to emerge are reduced.
Regardless of these approaches, any antimicrobial treatment selects for resistant species, and it is impossible to completely prevent the development of resistance. As the increasing prevalence of highly resistant microbes illustrates, there is a need to be vigilant about resistance and to investigate new treatment options. If bacteria become highly resistant and no medications are available to treat them, then infections that could have been easily treated can become dangerous or even lethal. Therefore, addressing antimicrobial resistance is a public health priority.
Because resistance develops rapidly, there is a constant search for new antimicrobials combined with updated recommendations and treatment guidelines.
To find new antimicrobials, scientists are examining soils and microbial products for antimicrobial activity using high-throughput screening methods that use automation to screen large numbers of samples simultaneously. Most microbes cannot be cultured in the laboratory, so one new approach is to grow microbes in the soil and use an iChip device to test for antimicrobials. The iChip device encloses bacteria, then can be placed in their natural environment so that substances can diffuse in and out to allow them to grow (Ling, 2020). By using the iChip, researchers can culture and study a wider range of species in many ways, including determining whether they produce substances with antimicrobial properties.
Researchers are also searching other environments for new types of antimicrobials. For example, there may be more marine microbes with antimicrobial properties. Some researchers are using combinatorial chemistry, in which many related compounds are made from simple compounds, to develop molecules that can be tested for antimicrobial activity.
There is also interest in developing ways to inhibit resistance mechanisms to older drugs. This could prolong the usefulness of existing medications. For example, some microbes exhibit resistance by breaking a ring found in penicillin and related medications, but new medications have been developed that inhibit the enzyme and therefore protect the ring.
Finally, there is interest in finding ways to slow the progress of infection by interfering with mechanisms that microbes use to cause infection. This would not kill the microbes but could give the immune system more time to respond and potentially prevent the infection from worsening.
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “MICROBIOLOGY.” ACCESS FOR FREE AT openstax.org/details/books/microbiology. LICENSE: CC ATTRIBUTION 4.0 INTERNATIONAL.
REFERENCES
Methicillin-resistant Staphylococcus aureus (MRSA). (2022). Center for Disease Control and Prevention. www.cdc.gov/mrsa/healthcare/outpatient.html
CDC Reminds Clinical Laboratories and Healthcare Infection Preventionists of their Role in the Search and Containment of Vancomycin-Resistant Staphylococcus aureus (VRSA). (2022). Center for Disease Control and Prevention.
www.cdc.gov/hai/settings/lab/vrsa_lab_search_containment.html
Hata Memorial Museum. (n.d.). German Dr Paul Ehrlich and Japanese Dr Sahachiro Hata. Wikimedia. https://commons.wikimedia.org/wiki/File:Paul_Ehrlich_and_Sahachiro_Hata.jpg
Ling, L., Schneider, T., Peoples, A. et al. A new antibiotic kills pathogens without detectable resistance. Nature, 517(7535), 455–459. doi.org/10.1038/nature14098