Table of Contents |
Gametogenesis in females is called oogenesis. The ovarian cycle governs the preparation of endocrine tissues and the release of eggs, and it includes two interrelated processes: oogenesis (the production of gametes) and folliculogenesis (the growth and development of ovarian follicles). You will learn more about oogenesis in this lesson and about folliculogenesis in a future lesson.
As happens with spermatogenesis, oogenesis involves the process of meiosis, and this process begins with ovarian stem cells called oogonia (singular oogonium), which are located in the outermost layers of the ovaries. In oogenesis, oogonia form during the embryological development of the individual. Oogonia divide via mitosis, much like spermatogonia in the testis. Unlike spermatogonia, however, oogonia undergo mitosis and form primary oocytes in the fetal ovary prior to birth (approximately one to two million oocytes by the time of birth).
During meiosis, two nuclear divisions separate the paired chromosomes in the nucleus and then separate the chromatids that were made during an earlier stage of the cell’s life cycle. Meiosis and its associated cell divisions produce haploid cells with half of each pair of chromosomes normally found in diploid cells.
Recall that oocytes are contained within the ovarian follicles. These primary oocytes in primordial follicles are then arrested (stop development) at prophase I of meiosis I. At the time of birth, all future eggs are in prophase I. They again resume development years later, beginning at puberty, and continue until the person is near menopause, which marks the cessation of a female's reproductive functions as the menstrual cycle ends.
Starting in adolescence, anterior pituitary hormones cause the development of a few follicles in an ovary each month. The initiation of ovulation—the release of an oocyte from the ovary—marks the transition from puberty into reproductive maturity. From then on, throughout the reproductive years, ovulation occurs approximately once every 28 days. Just prior to ovulation, a surge of luteinizing hormone (LH) triggers the resumption of meiosis in a primary oocyte. This results in a primary oocyte finishing the first meiotic division.
However, as you can see in the figure below, this cell division does not result in two identical cells. The cell divides unequally, with most of the cytoplasm and organelles going to one cell, called a secondary oocyte, and only one set of chromosomes and a small amount of cytoplasm going to the other cell. The larger cell, the secondary oocyte, eventually leaves the ovary during ovulation. The smaller cell, called the first polar body, remains within the ovary and may or may not complete meiosis and produce second polar bodies; ultimately, these cells will usually die and eventually disintegrate.
Cell division is again arrested in the secondary oocyte, this time at metaphase II. At ovulation, this secondary oocyte is released and travels toward the uterus through the oviduct. If the secondary oocyte is fertilized, the cell continues through meiosis II, producing a second polar body and haploid egg, which fuses with the haploid sperm to form a fertilized egg (zygote) containing all 46 chromosomes. Even though oogenesis produces up to four cells, only one survives.
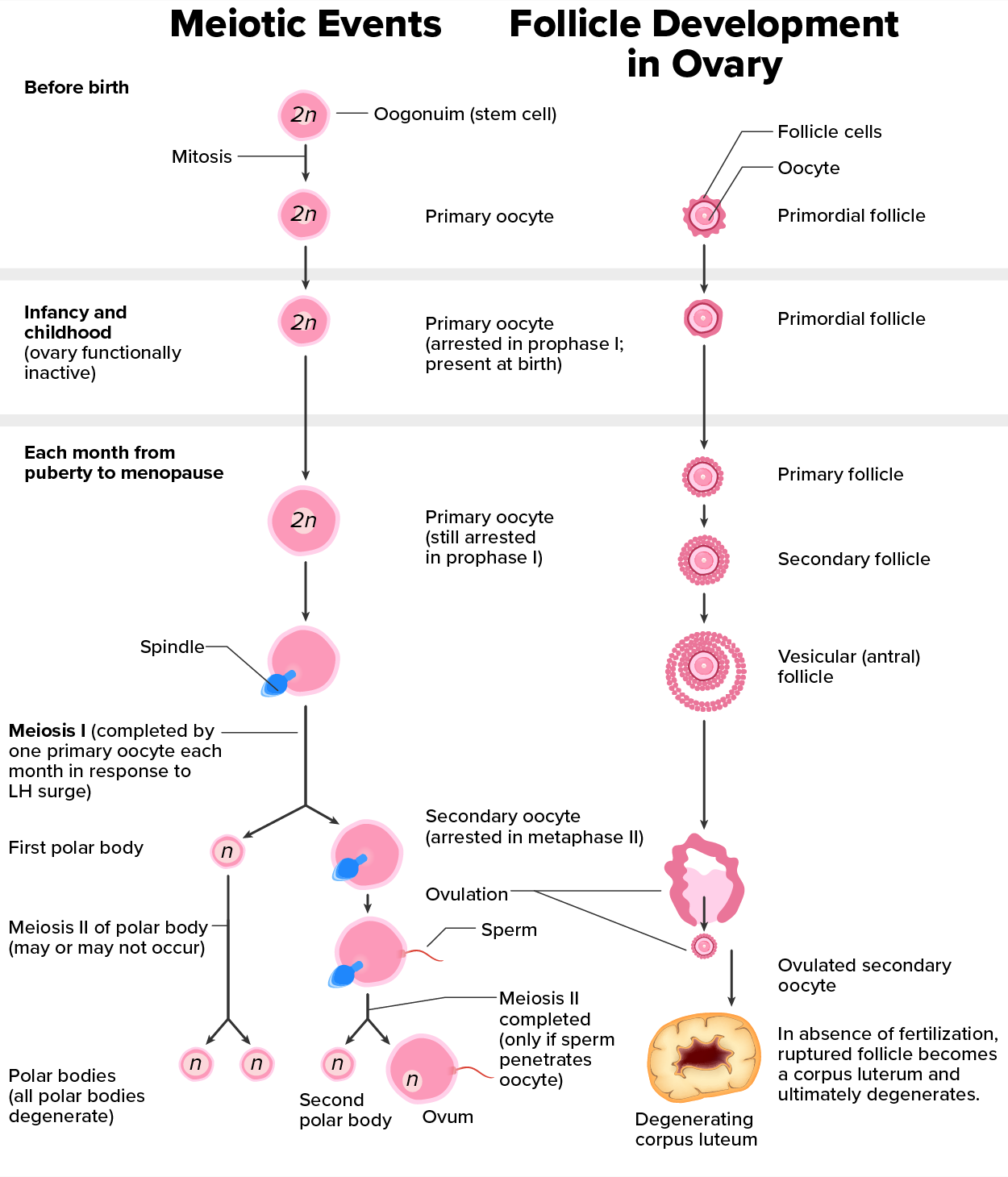
How does the diploid secondary oocyte become an ovum—the haploid female gamete? Meiosis of a secondary oocyte is completed only if a sperm succeeds in penetrating its barriers. Meiosis II then resumes, producing one haploid (“n”) ovum that, at the instant of fertilization by a (haploid) sperm, becomes the first diploid cell of the new offspring (a diploid, or “2n”, zygote). Thus, the ovum can be thought of as a brief, transitional, haploid stage between the diploid oocyte and diploid zygote.
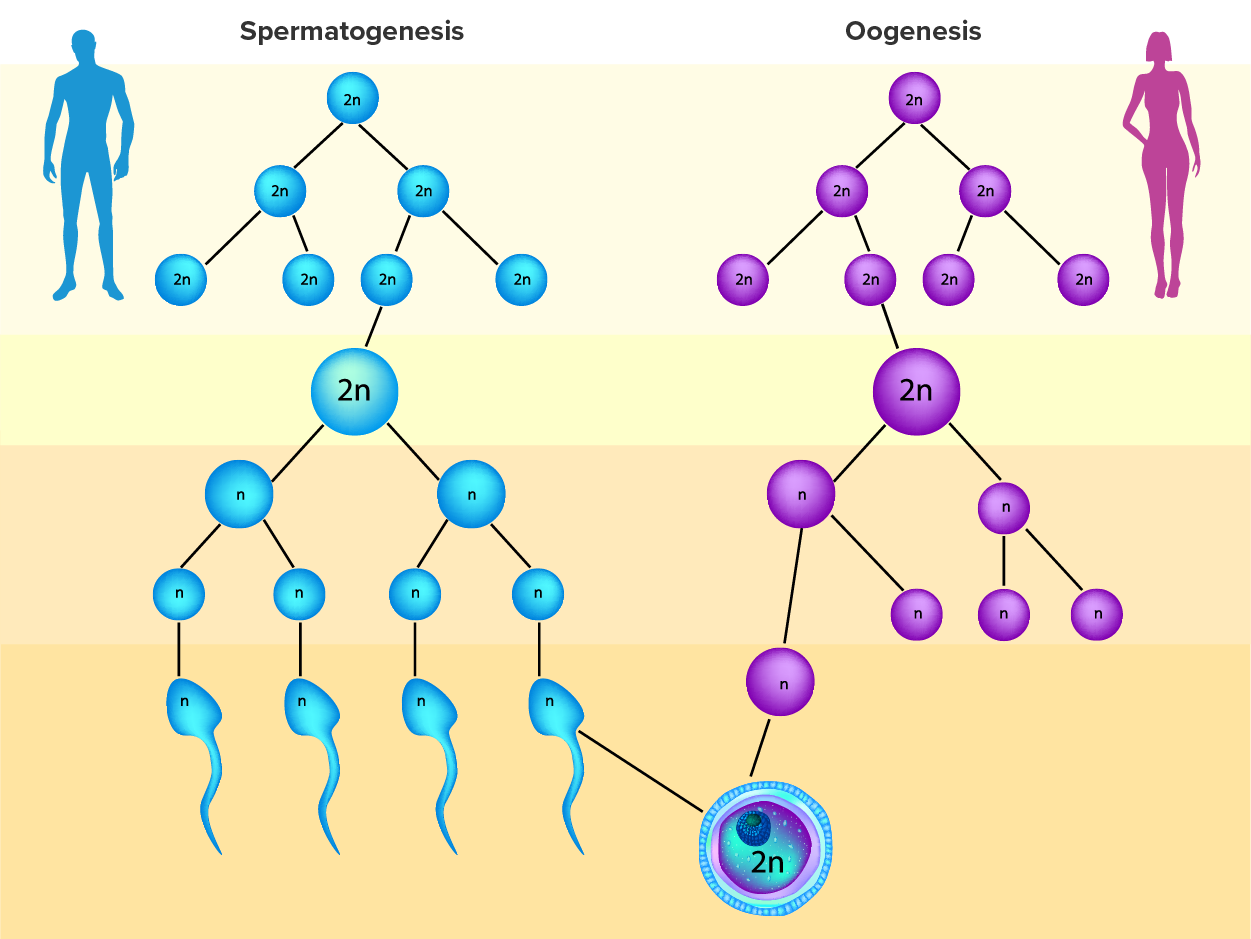
The larger amount of cytoplasm contained in the female gamete is used to supply the developing zygote with nutrients during the period between fertilization and implantation into the uterus. Interestingly, sperm contribute only DNA at fertilization—not cytoplasm. Therefore, the cytoplasm and all of the cytoplasmic organelles in the developing embryo are of egg-derived origin. This includes mitochondria, which contain their own DNA. Scientific research in the 1980s determined that mitochondrial DNA was maternally inherited, meaning that you can trace your mitochondrial DNA directly to your biological mother, her mother, and so on back through your female ancestors.
IN CONTEXT
Mapping Human History with Mitochondrial DNA
When we talk about human DNA, we are usually referring to nuclear DNA; that is, the DNA coiled into chromosomal bundles in the nucleus of our cells. We inherit half of our nuclear DNA from one parent, and half from the other parent. However, mitochondrial DNA (mtDNA) comes only from the mitochondria in the cytoplasm of the ovum we inherit from our mother. Our mother received mtDNA from their mother, who got it from their mother, and so on. Each of our cells contains approximately 1,700 mitochondria, with each mitochondrion packed with mtDNA containing approximately 37 genes.
Mutations (changes) in mtDNA occur spontaneously in a somewhat organized pattern at regular intervals in human history. By analyzing these mutational relationships, researchers have been able to determine that we can all trace our ancestry back to one female who lived in Africa about 200,000 years ago. More precisely, that human is our most recent common ancestor through matrilineal descent.
This doesn’t mean that everyone’s mtDNA today looks exactly like that of our common ancestor. Because of the spontaneous mutations in mtDNA that have occurred over the centuries, researchers can map different “branches” off of the “main trunk” of our mtDNA family tree. Your mtDNA might have a pattern of mutations that aligns more closely with one branch, and your neighbor’s may align with another branch. Still, all branches eventually lead back to the common ancestor.
But what happened to the mtDNA of all of the other Homo sapiens females who were living 200,000 years ago? Researchers explain that, over the centuries, their female descendants died childless or with only male children, and thus, their maternal line—and its mtDNA—ended.
During oogenesis, errors in meiosis can result in issues such as abnormal chromosome segregation and oocyte maturation arrest. These meiotic defects can lead to female infertility because of oocyte maturation arrest, increased aneuploidy (an abnormal number of chromosomes in a cell), and poor quality of female gametes. This can result in low rates of success for fertility procedures, such as in vitro fertilization and intracytoplasmic sperm injection, as well as early pregnancy loss.
A striking difference between oogenesis in the female and spermatogenesis in the male is that about 20% of oocytes have the wrong number of chromosomes, whereas only 3%–4% of sperm have the wrong number.
| Term | Pronunciation | Audio File |
|---|---|---|
| Oogenesis | o·eh·gen·e·sis |
|
| Oogonia | o·eh·go·ni·a |
|
| Oocyte | o·eh·cyte |
|
| Ovulation | ovu·la·tion |
|
| Ovum | o·vum |
|
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM (1) "ANATOMY AND PHYSIOLOGY 2E" ACCESS FOR FREE AT OPENSTAX.ORG/DETAILS/BOOKS/ANATOMY-AND-PHYSIOLOGY-2E. (2) "CONCEPTS OF BIOLOGY" ACCESS FOR FREE AT OPENSTAX.ORG/DETAILS/BOOKS/CONCEPTS-BIOLOGY. LICENSING (1 & 2): CREATIVE COMMONS ATTRIBUTION 4.0 INTERNATIONAL
REFERENCES
Solovova, O. A., & Chernykh, V. B. (2022). Genetics of oocyte maturation defects and early embryo development arrest. Genes, 13(11), 1920.