Table of Contents |
Visible light and other forms of electromagnetic radiation, or energy transmitted by waves that have both an electric-field and a magnetic-field component, play important roles in chemistry since they can be used to infer the energies of electrons within atoms and molecules. Much of modern technology is based on electromagnetic radiation.
EXAMPLE
Radio waves from a mobile phone, X-rays used by dentists, the energy used to cook food in your microwave, the radiant heat from red-hot objects, and the light from your television screen are all forms of electromagnetic radiation that exhibit wavelike behavior.A wave is an oscillation or periodic movement that can transport energy from one point in space to another. Common examples of waves are all around us. Shaking the end of a rope transfers energy from your hand to the other end of the rope, dropping a pebble into a pond causes waves to ripple outward along the water's surface, and the expansion of air that accompanies a lightning strike generates sound waves (thunder) that can travel outward for several miles. In each of these cases, kinetic energy is transferred through matter (the rope, water, or air) while the matter remains essentially in place.
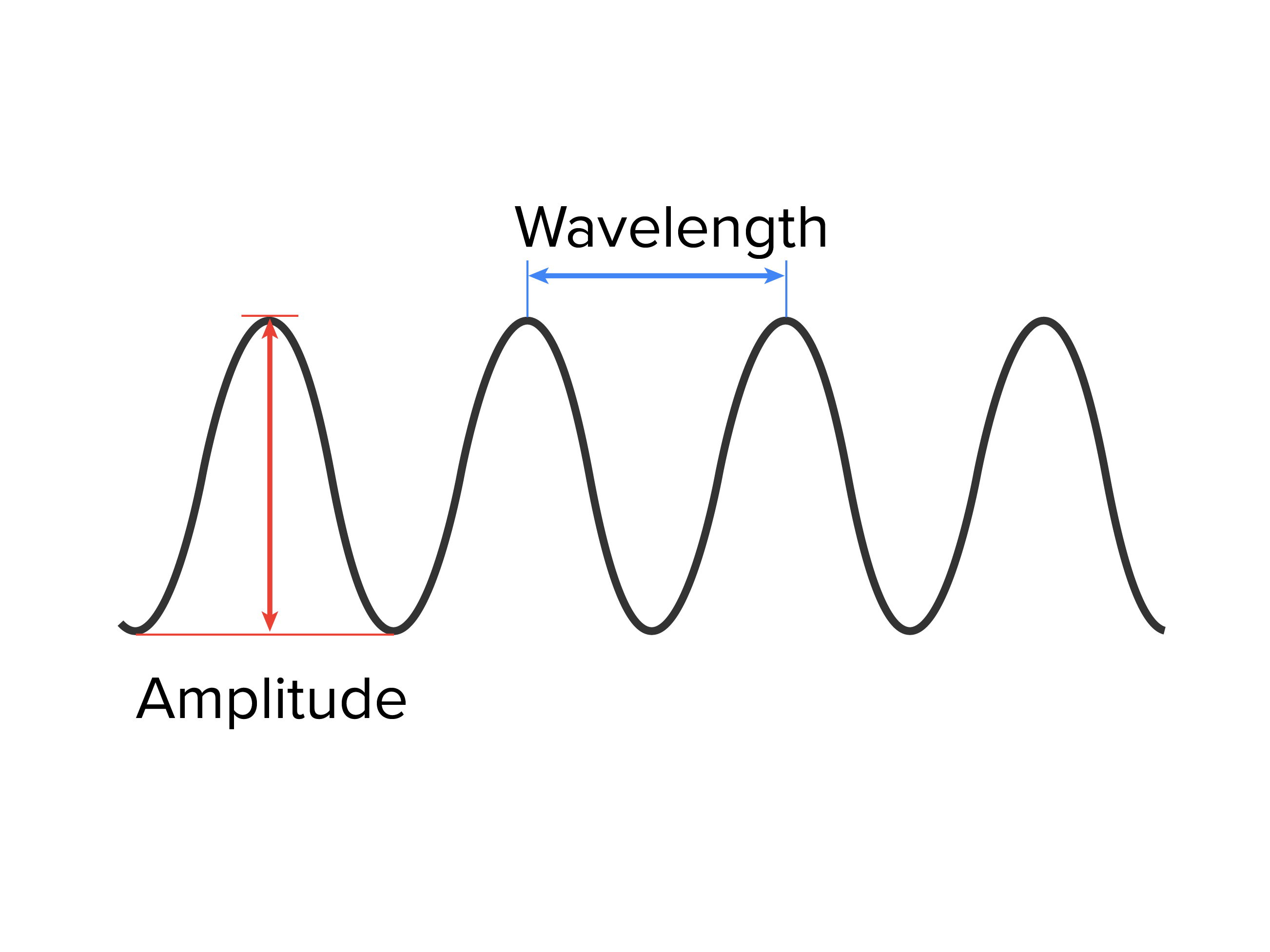
All waves, including forms of electromagnetic radiation, are characterized by a wavelength (denoted by λ, the lowercase Greek letter lambda), a frequency (denoted by 𝜈, the lowercase Greek letter nu), and an amplitude. The wavelength is the distance between two consecutive peaks or troughs in a wave (measured in meters). Electromagnetic waves have wavelengths that fall within an enormous range—wavelengths of kilometers (10 m) to picometers (10
m) to picometers (10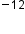 m) have been observed.
m) have been observed.
The frequency is the number of wave cycles that pass a specified point in space in a specified amount of time (measured in seconds). A cycle corresponds to one complete wavelength. The unit for frequency expressed as cycles per second [s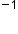 ], is the hertz (Hz). The amplitude corresponds to the magnitude of the wave's displacement, and so this corresponds to one-half the height between the peaks and troughs. The amplitude is related to the intensity of the wave, which for light is brightness, and for sound is loudness.
], is the hertz (Hz). The amplitude corresponds to the magnitude of the wave's displacement, and so this corresponds to one-half the height between the peaks and troughs. The amplitude is related to the intensity of the wave, which for light is brightness, and for sound is loudness.
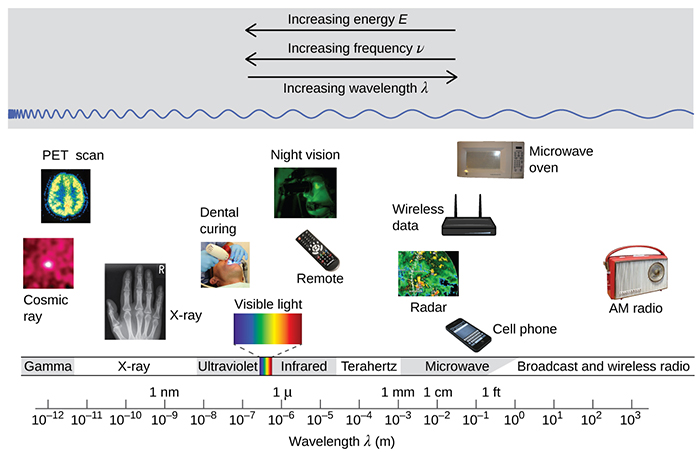
Wavelength and frequency are inversely proportional. As the wavelength increases, the frequency decreases, and vice versa. The electromagnetic spectrum is the range of all types of electromagnetic radiation. Each of the various colors of visible light has specific frequencies and wavelengths associated with them, and you can see that visible light makes up only a small portion of the electromagnetic spectrum. Because the technologies developed to work in various parts of the electromagnetic spectrum are different—for reasons of convenience and historical legacies—different units are typically used for different parts of the spectrum. For example, radio waves are usually specified as frequencies (typically in units of MHz), while the visible region is usually specified in wavelengths (typically in units of nm or angstroms).
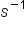 ].
].Following the work of Ernest Rutherford and his colleagues in the early twentieth century, the picture of atoms consisting of tiny dense nuclei surrounded by lighter and even tinier electrons continually moving about the nucleus was well established. This picture was called the planetary model since it pictured the atom as a miniature “solar system” with the electrons orbiting the nucleus like planets orbiting the sun. The simplest atom is hydrogen, consisting of a single proton as the nucleus about which a single electron moves. The electrostatic force attracting the electron to the proton depends only on the distance between the two particles.
When Bohr calculated his theoretical value and compared it with the experimentally accepted constant, he got excellent agreement. Since this constant was one of the most precisely measured constants at that time, this level of agreement was astonishing and meant that Bohr’s model was taken seriously, despite the many assumptions that Bohr needed to derive it. The Bohr model of the atom consisted of a small, dense nucleus surrounded by orbiting electrons.
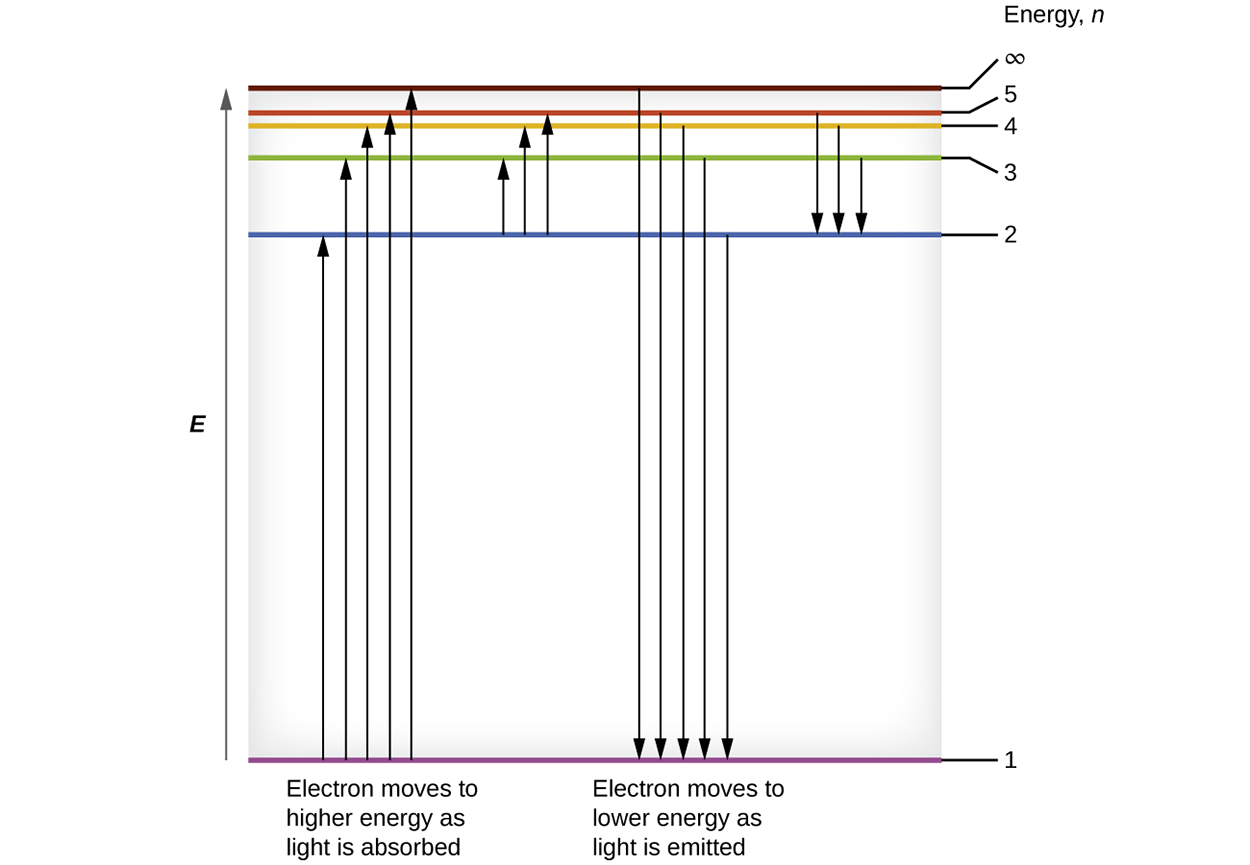
The lowest few energy levels are shown below. One of the fundamental laws of physics is that matter is most stable with the lowest possible energy. Thus, the electron in a hydrogen atom usually moves in the first energy level, the level which has the lowest energy. When the electron is in this lowest energy level, the atom is said to be in its ground electronic state (or simply ground state).
If the atom receives energy from an outside source, it is possible for the electron to move to a higher energy level and the atom is now in an excited electronic state (or simply an excited state) with higher energy. When an electron transition’s from an excited state (higher energy level) to a less excited state or ground state, the difference in energy is emitted as a photon. Similarly, if a photon is absorbed by an atom, the energy of the photon moves an electron from a lower energy level up to a more excited one.
The equation also shows us that as the electron’s energy increases, the electron is found at greater distances from the nucleus. This is implied by the inverse dependence of electrostatic attraction on distance, since, as the electron moves away from the nucleus, the electrostatic attraction between it and the nucleus decreases and it is held less tightly in the atom.
Unfortunately, despite Bohr’s remarkable achievement, he was unable to extend his theory to the next simplest atom, Helium (He), which only has two electrons. Bohr’s model was severely flawed since it was still based on classical mechanics, which is unusable in the microscopic domain. To understand the microscopic domain, a new field of math named quantum mechanics was developed to supersede classical mechanics. Quantum mechanics is a topic beyond the scope of this course.
In the image below the horizontal lines show the relative energy of orbits in the Bohr model of the hydrogen atom, and the vertical arrows depict the energy of photons absorbed (left) or emitted (right) as electrons move between these orbits. The largest energy difference is between the ground state and the first energy level. The energy difference between the second and third energy levels is much smaller than between the first and the second, and the energy difference keeps getting smaller between higher energy levels (3 and 4, 4 and 5, etc.).
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “CHEMISTRY: ATOMS FIRST 2E”. ACCESS FOR FREE AT Chemistry: Atoms First 2e. LICENSE: CREATIVE COMMONS ATTRIBUTION 4.0 INTERNATIONAL