Table of Contents |
The Gram staining procedure is a common and important type of differential staining. It classifies bacteria into one or two groups based on the characteristics of their cell walls. This procedure was developed by Danish microbiologist Hans Christian Gram in 1884.
Cells that retain crystal violet and remain purple are called gram-positive, and cells that lose crystal violet and become pink when dyed with safranin are called gram-negative. These differences reflect the differences in the peptidoglycan layer, but there are other important differences between gram-positive and gram-negative cells. You will learn more about these in the lessons about prokaryotic cells and the characteristics of taxonomic groups of bacteria.
IN CONTEXT
There are several considerations to remember when using the Gram staining procedure. First, it is important to use young, healthy colonies because older bacterial cells may have damaged cell walls that do not retain the primary stain as well as healthy cells do. Second, errors such as leaving the decolorizer on too long can affect the result. In some cases, most cells will appear gram-positive, while a few will appear to be gram-negative. This suggests damage to the individual cells or that the decolorizer was left on too long. The cells should still be classified as gram-positive if they are all the same species.
Besides their differing interactions with dyes and decolorizing agents, the chemical differences between gram-positive and gram-negative cells have implications with clinical relevance. Therefore, Gram staining is a valuable clinical tool, and knowing how to use it is an important skill.
EXAMPLE
Gram staining can help clinicians classify bacterial pathogens in a sample into categories associated with specific properties. This can be helpful in selecting an appropriate antibiotic. You will learn more about the characteristics of gram-positive and gram-negative bacteria and about antibiotics in other lessons.| Gram Staining Process | ||||
|---|---|---|---|---|
| Gram Staining Steps | Cell Effects | Gram-Positive | Gram-Negative | |
|
Step 1 Crystal violet The primary stain is added to the specimen smear. |
The cells are stained purple or blue. |
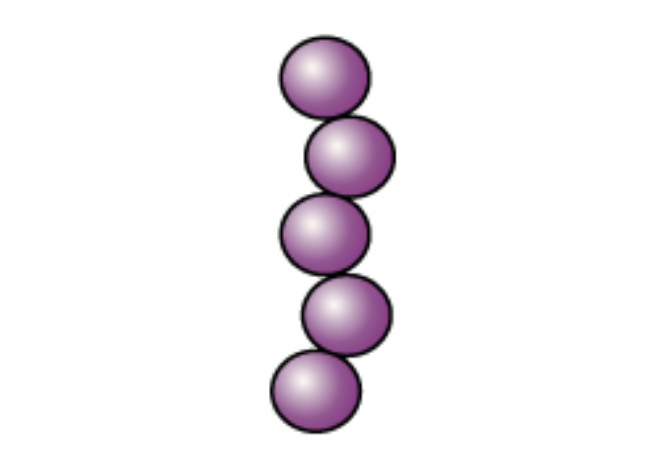
|
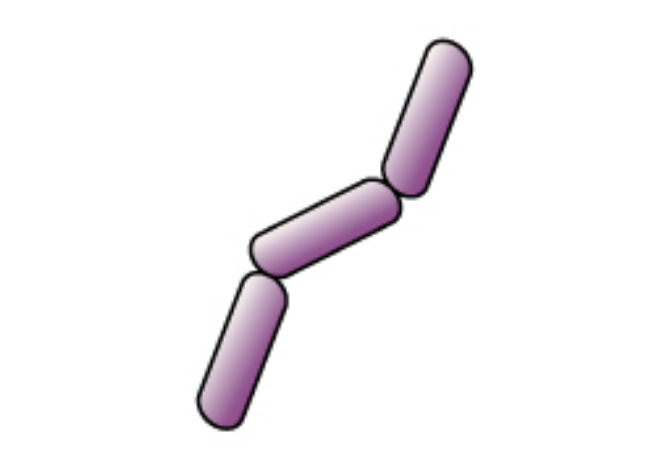
|
|
|
Step 2 Iodine The mordant makes the dye less soluble, so it adheres to cell walls. |
The cells remain purple or blue. |
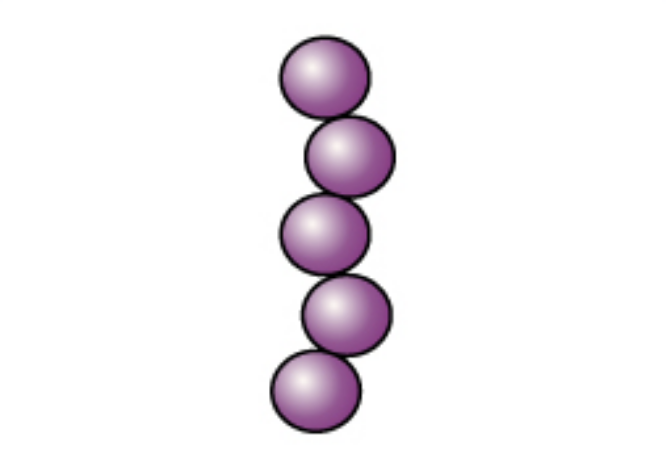
|
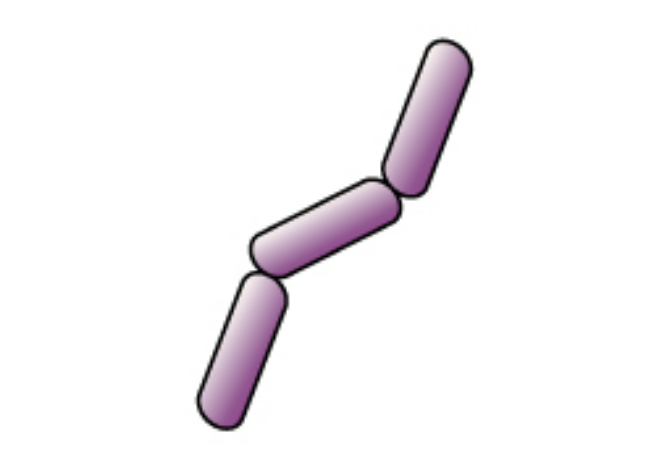
|
|
|
Step 3 Alcohol/Acetone The decolorizer washes away the stain from gram-negative cell walls. |
Gram-positive cells remain purple or blue. Gram-negative cells are colorless. |
|
|
|
|
Step 4 Safranin The counterstain allows dye adherence to gram-negative cells. |
Gram-positive cells remain purple or blue. Gram-negative cells appear pink or red. |

|
|
|
In the specimen shown in the image below, the gram-positive bacterium Staphylococcus aureus retains the crystal violet dye even after the decolorizing agent is added. Gram-negative Escherichia coli, the most common Gram stain quality-control bacterium, is decolorized and is only visible after the addition of the pink counterstain safranin.
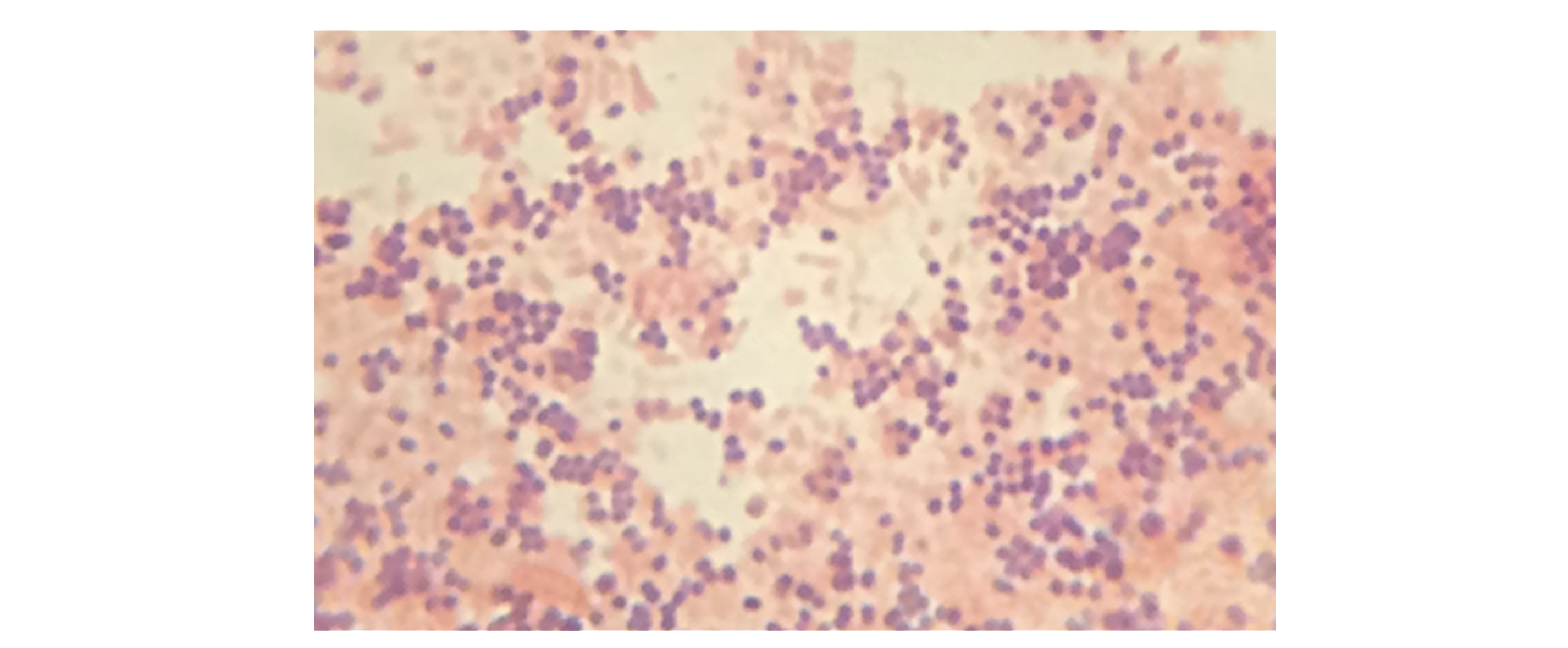
EXAMPLE
Gram staining is often used as a first step in identifying bacteria in the lab. Knowing whether a bacterial infection is gram-positive or gram-negative can be helpful in choosing an effective antibiotic.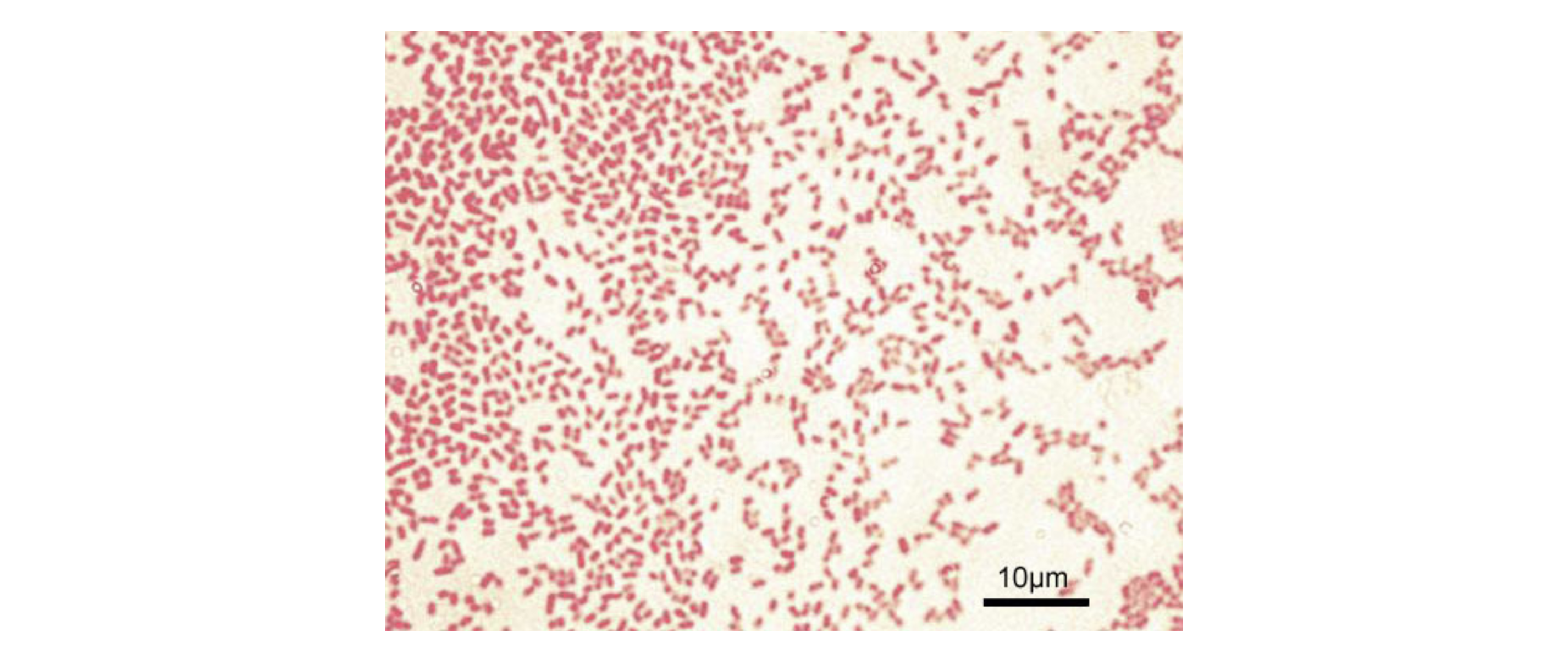
Acid-fast staining differentiates between two types of gram-positive cells: those that have waxy mycolic acid in their cell walls and those that do not. It is another commonly used, differential staining technique that can be an important diagnostic tool.
Two major techniques for acid-fast staining are the Ziehl-Neelsen technique and the Kinyoun technique. Both use carbolfuchsin as the primary stain. The waxy, acid-fast cells retain the carbolfuchsin and appear red or pink even after a decolorizing agent (an acid-alcohol solution) is added. A secondary counterstain, methylene blue, is used and this stains non-acid-fast cells blue. The techniques differ in that the Ziehl-Neelsen method uses heat to infuse the carbolfuchsin and the Kinyoun technique does not.
Acid-fast staining is used to differentiate red or pink acid-fast bacteria (AFB) from blue non-acid-fast bacteria. This distinction can have clinical importance.
EXAMPLE
Acid-fast staining is important clinically to detect AFB such as Mycobacterium tuberculosis, which causes tuberculosis.In the micrograph below, Ziehl-Neelsen staining has rendered these M. tuberculosis cells red and the surrounding growth indicator medium blue.
Some bacterial cells can form endospores that allow them to survive harsh conditions while remaining dormant. You will learn more about endospores in other lessons. They are particularly difficult to kill and important to consider when trying to remove or kill microbes that may cause harm.
Endospore staining uses two stains to differentiate endospores from the rest of the cell. The most commonly used approach is the Schaeffer-Fulton method. The primary stain, malachite green, is pushed by heat into the endospore. Next, water is used to decolorize the cell, while the endospore remains green. A safranin counterstain is used. If endospores are present, they will be stained green whether they are inside or outside of a cell. The vegetative (growing) cells will be stained pink.
Endospores are formed by some pathogens of considerable clinical significance.
IN CONTEXT
Important pathogens that form endospores include the following:
Bacillus anthracis (causes anthrax and raises concern as a possible bioterrorism agent)
Clostridium tetani (causes tetanus)
C. botulinum (causes botulism)
Clostridiodes (Clostridium) difficile (causes a potentially severe intestinal infection)
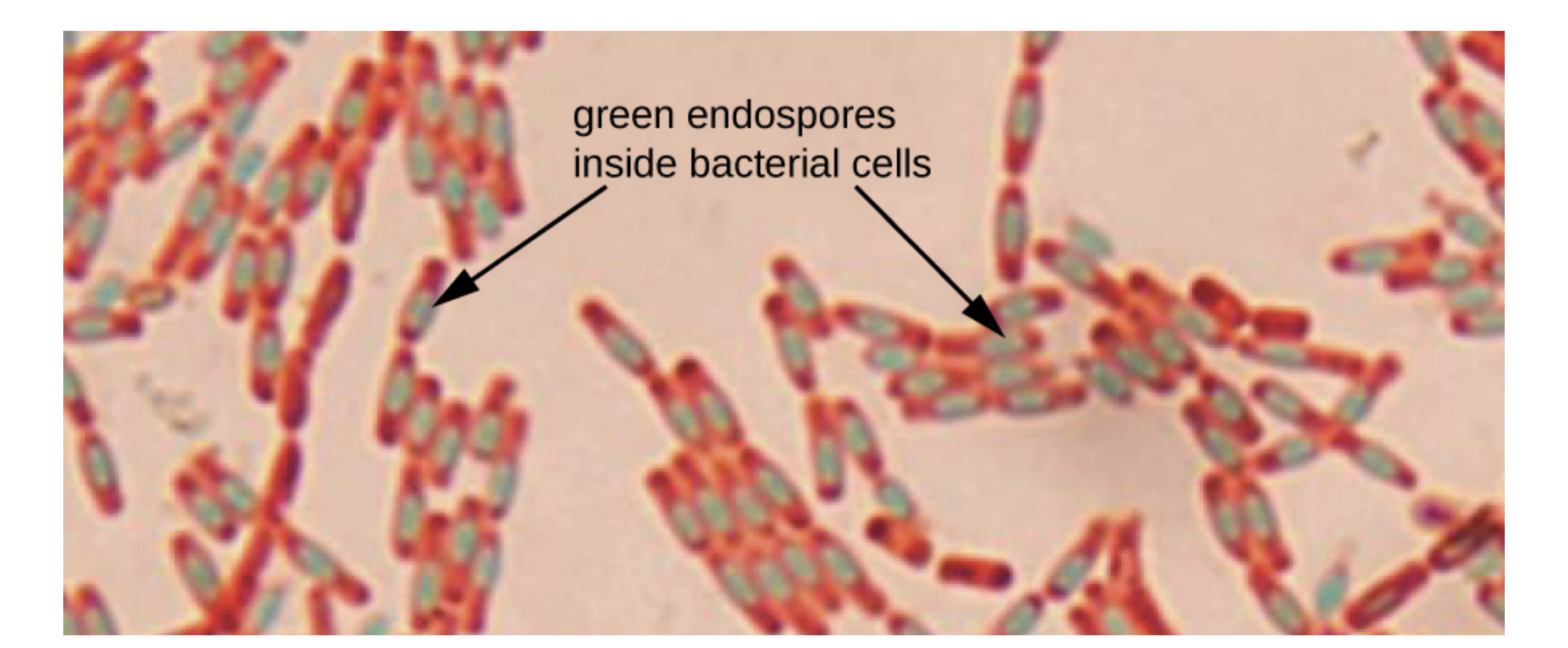
The micrograph below is a stained preparation of Bacillus subtilis with the endospores appearing green and the vegetative cells pink.
Many organisms use long, thin structures called flagella for movement. These structures are different in Bacteria, Archaea, and Eukarya and are called archaella in Archaea. Flagella are very thin, which makes them difficult to see under a light microscope unless specialized flagella staining techniques are used.
Flagella staining thickens the flagella by applying a mordant that thickens the flagella. The mordant is generally tannic acid, but sometimes potassium alum is also used. Next, the specimen is stained with pararosaniline (most commonly) or basic fuchsin.
This approach is often used by microbiologists to aid in understanding, classifying, and identifying bacteria. However, it is not commonly used in clinical settings.
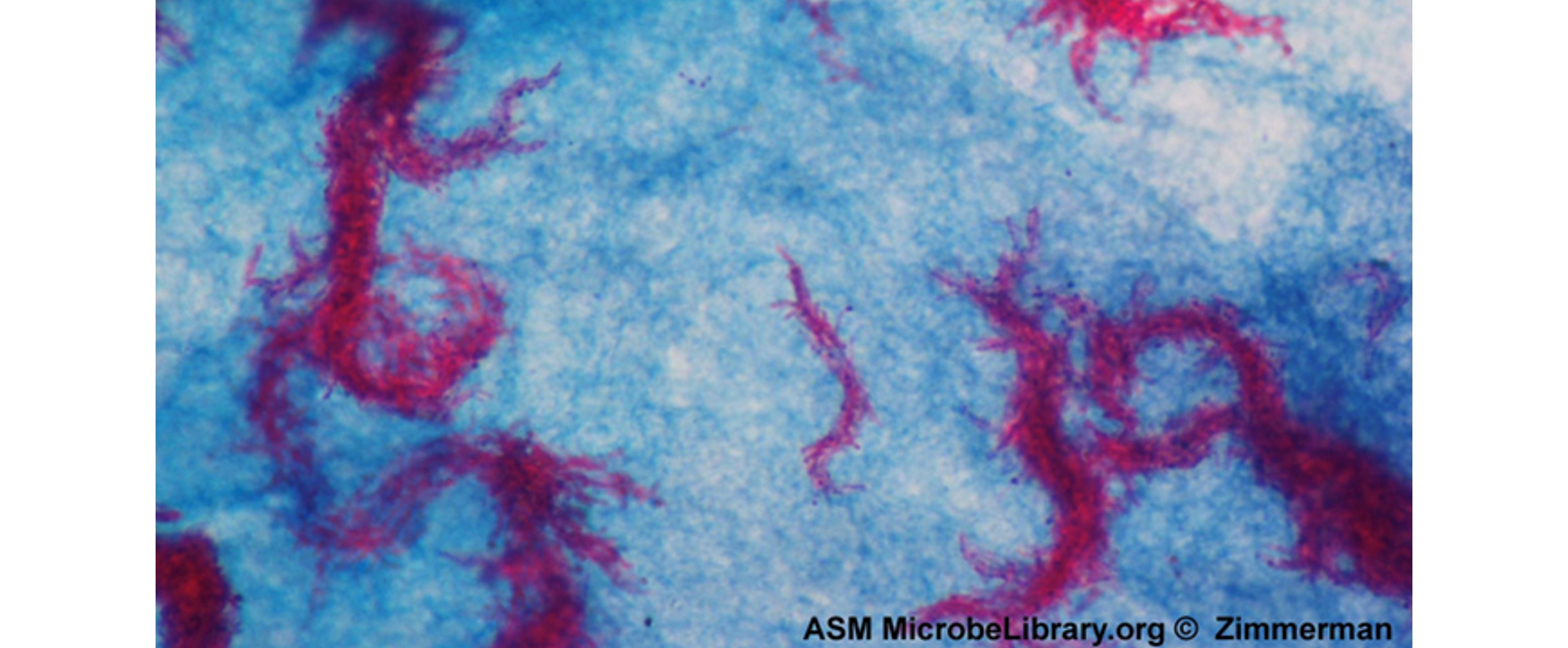
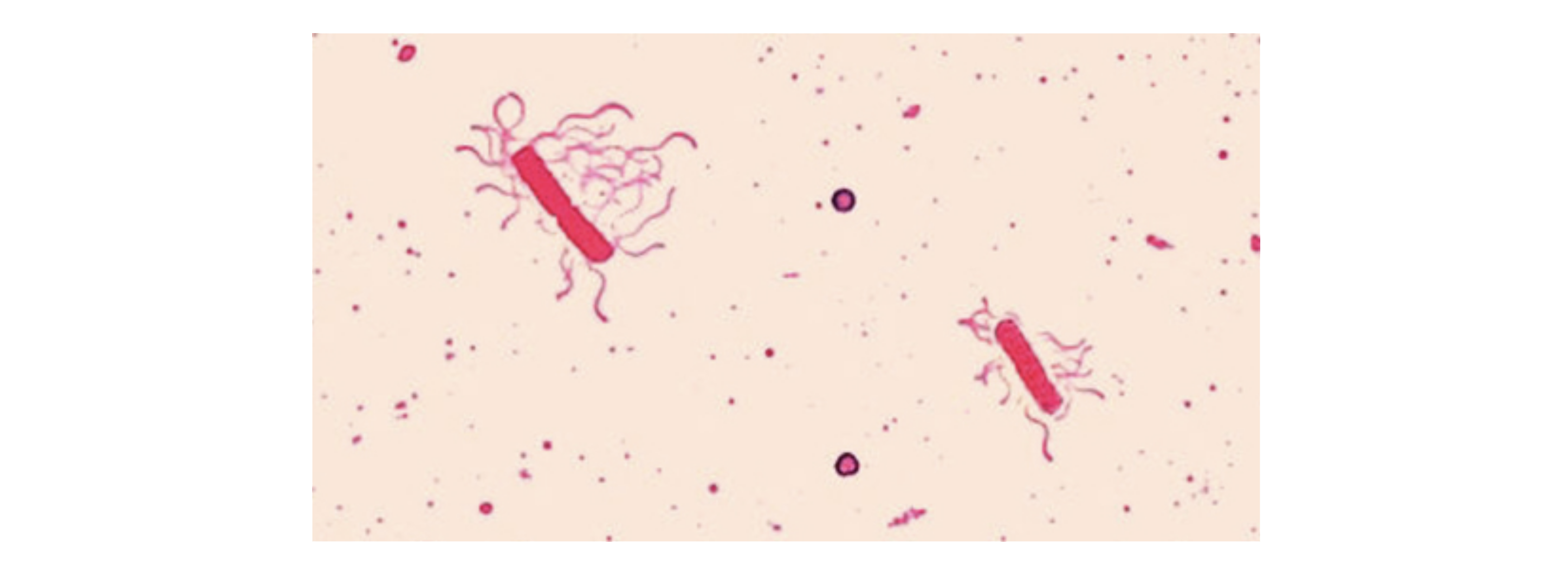
To stain flagella, it is important to treat specimens carefully. Flagella are delicate and can be easily broken off, as shown in the image below of a flagella stain of Bacillus cereus, a common cause of foodborne illness. The stain reveals that the cells have numerous flagella, which are used for locomotion.
Certain bacteria and yeasts have a protective outer structure called a capsule. A capsule is an organized structure found outside of the cell wall. The capsule may be associated with increased virulence (ability to cause disease). You will learn more about capsules in other tutorials, including the lesson on prokaryotic cells.
Because capsules do not absorb most basic dyes, capsule staining generally involves a negative staining technique. The dye stains the background but is not taken up by the capsules. This produces a clear region where the capsule is located.
One common negative staining technique used to visualize capsules involves adding a few drops of India ink or nigrosin to a specimen. The specimen does not need to be fixed. A positive stain may also be used to stain the cell, leaving a clear halo indicating the location of the capsule.
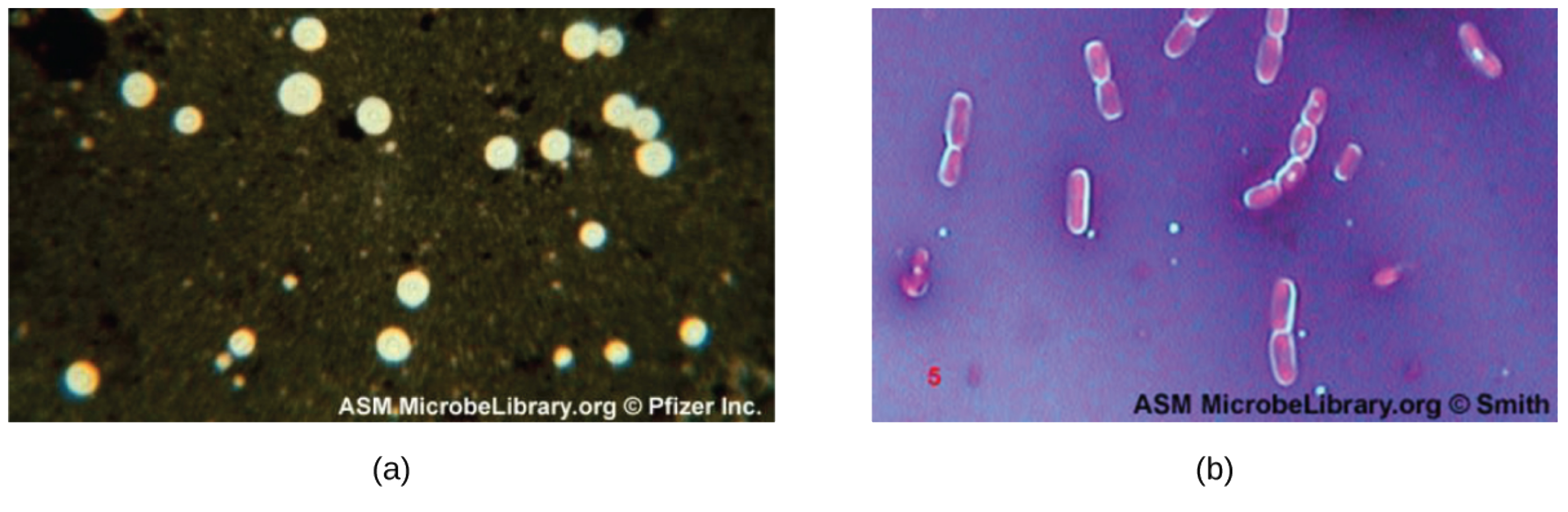
In the micrograph image (a) below, India ink was used to stain the background around the cells of the yeast Cryptococcus neoformans. The halos surrounding the cells are the polysaccharide capsules. In image (b), crystal violet and copper sulfate dyes cannot penetrate the encapsulated Bacillus cells in this negatively stained sample. The encapsulated cells appear to have a light-blue halo.
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “MICROBIOLOGY.” ACCESS FOR FREE AT openstax.org/details/books/microbiology. LICENSE: CC ATTRIBUTION 4.0 INTERNATIONAL.
REFERENCES
Beeby, M., Ferreira, J. L., Tripp, P., Albers, S. V., & Mitchell, D. R. (2020). Propulsive nanomachines: the convergent evolution of archaella, flagella and cilia. FEMS Microbiology reviews, 44(3), 253–304. doi.org/10.1093/femsre/fuaa006
Khan, S., & Scholey, J. M. (2018). Assembly, Functions and Evolution of Archaella, Flagella and Cilia. Current Biology : CB, 28(6), R278–R292. doi.org/10.1016/j.cub.2018.01.085
Merriam-Webster. (n.d.). Motility. In Merriam-Webster.com Dictionary. Retrieved August 1, 2022, from www.merriam-webster.com/dictionary/motility
Parker, N., Schneegurt, M., Thi Tu, A.-H., Lister, P., & Forster, B. (2016). Microbiology. OpenStax. Access for free at openstax.org/books/microbiology/pages/1-introduction