Table of Contents |
The formation of a solution is an example of a spontaneous process, a process that occurs under specified conditions without the requirement of energy from some external source. Sometimes a mixture is stirred to speed up the dissolution process, but this is not necessary; a homogeneous solution will form eventually.
The process of a solute (as a liquid, solid, or gas) dissolving in a solvent is called dissolution. Three types of intermolecular attractive forces are relevant to the dissolution process: solute-solute, solvent-solvent, and solute-solvent.
As illustrated below, the formation of a solution may be viewed as a stepwise process in which energy is consumed to overcome solute-solute and solvent-solvent attractions, which are endothermic processes. Energy is released when solute-solvent attractions are established, which is an exothermic process referred to as solvation. Solvation is the interaction of a solvent with its dissolved solutes.
The relative magnitudes of the energy changes associated with these stepwise processes determine whether the dissolution process overall will release or absorb energy. In some cases, solutions do not form because the energy required to separate solute and solvent species is so much greater than the energy released by solvation.
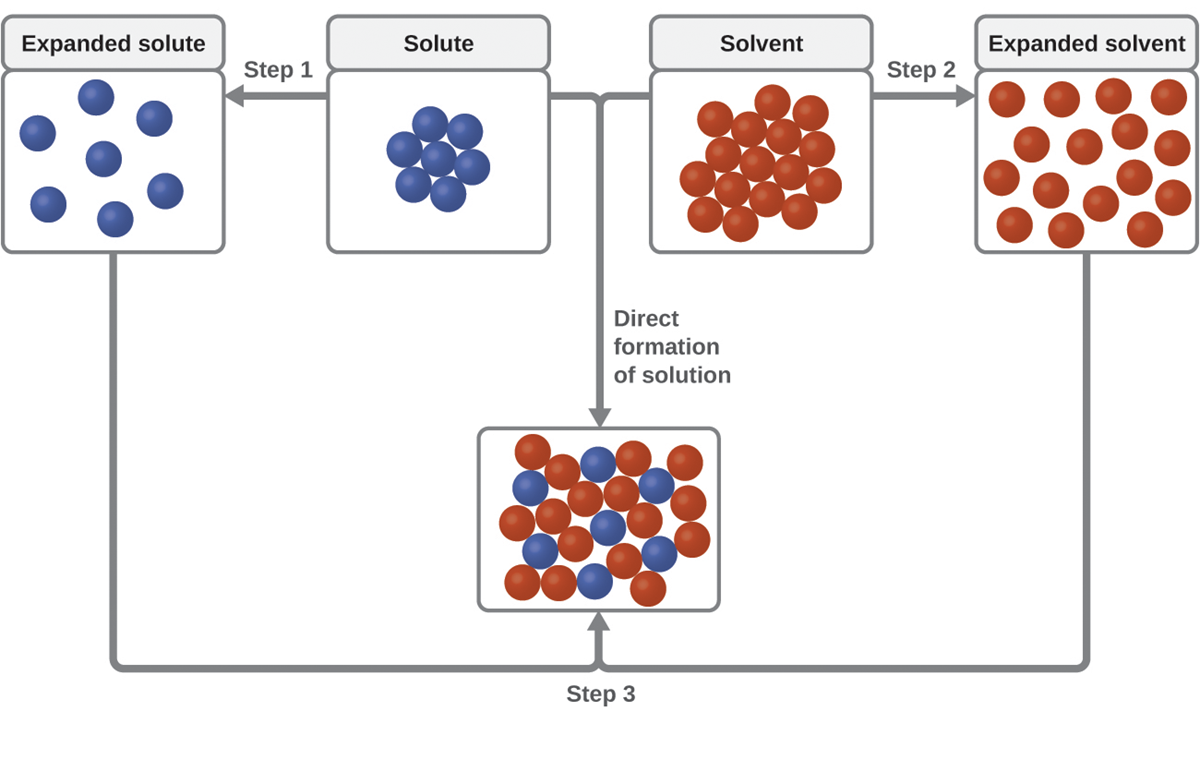
This schematic representation of dissolution in the above image shows a stepwise process involving the endothermic separation of solute and solvent species (Steps 1 and 2) and exothermic solvation (Step 3).
EXAMPLE
Consider the example of an ionic compound dissolving in water. Formation of the solution requires the electrostatic forces between the cations and anions of the compound (solute-solute) to be overcome completely as attractive forces are established between these ions and water molecules (solute-solvent). Hydrogen bonding between a relatively small fraction of the water molecules must also be overcome to accommodate any dissolved solute.If the solute’s electrostatic forces are significantly greater than the solvation forces, the dissolution process is significantly endothermic, and the compound may not dissolve to an appreciable extent. Calcium carbonate, the major component of coral reefs, is one example of such an “insoluble” ionic compound.
On the other hand, if the solvation forces are much stronger than the compound’s electrostatic forces, the dissolution is significantly exothermic, and the compound may be highly soluble. A common example of this type of ionic compound is sodium chloride, commonly known as table salt.
As noted at the beginning of this section, spontaneous solution formation is favored, but not guaranteed, by exothermic dissolution processes. While many soluble compounds do, indeed, dissolve with the release of heat, some dissolve endothermically.
EXAMPLE
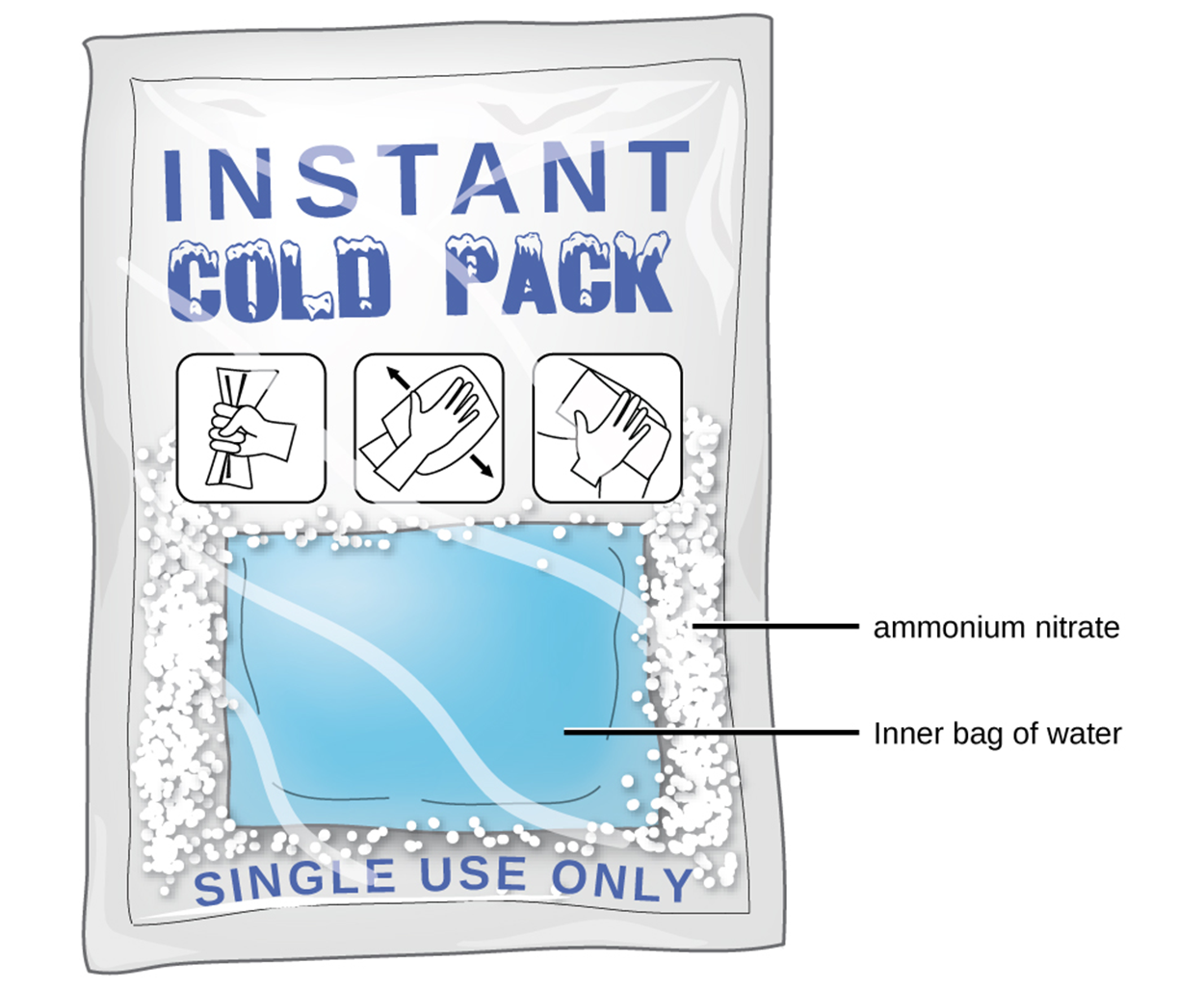
Ammonium nitrate (NH4NO3) is one such example and is used to make instant cold packs, like the one pictured below, which are used for treating injuries. A thin-walled plastic bag of water is sealed inside a larger bag with solid NH4NO3. When the smaller bag is broken, a solution of NH4NO3 forms, absorbing heat from the surroundings (the injured area to which the pack is applied) and providing a cold compress that decreases swelling.Endothermic dissolutions such as this one require greater energy input to separate the solute species than is recovered when the solutes are solvated, but they are spontaneous nonetheless due to the increase in disorder that accompanies the formation of the solution.
In the previous section, the process of a solute dissolving into a solution was described. Some compounds (such as calcium carbonate, CaCO3) are held together so tightly (solute-solute interactions) that they are energetically not favorable. In other words, it takes so much energy to break apart the calcium carbonate compared to the energy gain from forming a solution, that only a very small amount of the calcium carbonate will actually dissolve in water (no matter how much water you have).
If you want to prove this for yourself, get a piece of chalk (calcium carbonate) and try to dissolve it in a beaker of water. No matter how much you break the chalk or stir it, chalk will barely dissolve in water.
EXAMPLE
Imagine adding a small amount of sugar to a glass of water, stirring until all the sugar has dissolved, and then adding a bit more. You can repeat this process until the sugar concentration of the solution reaches its natural limit, a limit determined primarily by the relative strengths of the solute-solute, solute-solvent, and solvent-solvent attractive forces discussed in the previous section.You can be certain that you have reached this limit because, no matter how long you stir the solution, undissolved sugar remains. The concentration of sugar in the solution at this point is known as its solubility. The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when the dissolution process is at equilibrium.
When a solute’s concentration is equal to its solubility, the solution is said to be saturated with that solute. If the solute’s concentration is less than its solubility, the solution is said to be unsaturated. A solution that contains a relatively low concentration of solute is called dilute, and one with a relatively high concentration is called concentrated.
Solutions may be prepared in which a solute concentration exceeds its solubility. Such solutions are said to be supersaturated, and they are interesting examples of nonequilibrium states. For example, the carbonated beverage in an open container that has not yet “gone flat” is supersaturated with carbon dioxide gas; given time, the CO2 concentration will decrease until it reaches its solubility.
As for any solution, the solubility of a gas in a liquid is affected by the intermolecular attractive forces between solute and solvent species. Unlike solid and liquid solutes, however, there is no solute-solute intermolecular attraction to overcome when a gaseous solute dissolves in a liquid solvent since the atoms or molecules comprising a gas are far separated and experience negligible interactions.
Temperature is another factor affecting solubility, with gas solubility typically decreasing as temperature increases. This inverse relation between temperature and dissolved gas concentration is responsible for one of the major impacts of thermal pollution in natural waters.
When the temperature of a river, lake, or stream is raised, the solubility of oxygen in the water is decreased. Decreased levels of dissolved oxygen may have serious consequences for the health of the water’s ecosystems and, in severe cases, can result in large-scale death in fish populations.

The above image (a) shows the small bubbles of air in this glass of chilled water formed when the water warmed to room temperature and the solubility of its dissolved air decreased. (b) The decreased solubility of oxygen in natural waters subjected to thermal pollution can result in large-scale death in fish populations.
The solubility of a gaseous solute is also affected by the partial pressure of solute in the gas to which the solution is exposed. Gas solubility increases as the pressure of the gas increases. Carbonated beverages provide a nice illustration of this relationship. The carbonation process involves exposing the beverage to relatively high pressure of carbon dioxide gas and then sealing the beverage container, thus saturating the beverage with CO2 at this pressure.
When the beverage container is opened, a familiar hiss is heard as the carbon dioxide gas pressure is released, and some of the dissolved carbon dioxide is typically seen leaving solution in the form of small bubbles. At this point, the beverage is supersaturated with carbon dioxide and, with time, the dissolved carbon dioxide concentration will decrease to its equilibrium value and the beverage will become “flat.”

The above image demonstrates that opening a bottle of carbonated beverage reduces the pressure of the gaseous carbon dioxide above the beverage. The solubility of CO2 is thus lowered, and some dissolved carbon dioxide may be seen leaving the solution as small gas bubbles.
Some liquids may be mixed in any proportions to yield solutions; in other words, they have infinite mutual solubility and are said to be miscible.
EXAMPLE
Ethanol, sulfuric acid, and ethylene glycol (popular for use as antifreeze) are examples of liquids that are completely miscible with water. Two-cycle motor oil is miscible with gasoline, mixtures of which are used as lubricating fuels for various types of outdoor power equipment (chainsaws, leaf blowers, and so on).Miscible liquids are typically those with very similar polarities. Consider, for example, liquids that are polar or capable of hydrogen bonding. For such liquids, the dipole-dipole attractions (or hydrogen bonding) of the solute molecules with the solvent molecules are at least as strong as those between molecules in the pure solute or in the pure solvent. Hence, the two kinds of molecules mix easily.
Likewise, nonpolar liquids are miscible with each other because there is no appreciable difference in the strengths of solute-solute, solvent-solvent, and solute-solvent intermolecular attractions. The solubility of polar molecules in polar solvents and of nonpolar molecules in nonpolar solvents is, again, an illustration of the chemical axiom “like dissolves like.”
Two liquids that do not mix to an appreciable extent are called immiscible. Separate layers are formed when immiscible liquids are poured into the same container.
EXAMPLE
Gasoline, oil, benzene, carbon tetrachloride, some paints, and many other nonpolar liquids are immiscible with water.Relatively weak attractive forces between the polar water molecules and the nonpolar liquid molecules are not adequate to overcome much stronger hydrogen bonding between water molecules. The distinction between immiscibility and miscibility is really one of extent, so that miscible liquids are of infinite mutual solubility, while liquids said to be immiscible are of very low (though not zero) mutual solubility.

The dependence of solubility on temperature for a number of solids in water indicates a general trend of increasing solubility with temperature, although there are exceptions. The temperature dependence of solubility can be exploited to prepare supersaturated solutions of certain compounds. A solution may be saturated with the compound at an elevated temperature (where the solute is more soluble) and subsequently cooled to a lower temperature without precipitating the solute.
The formation of a supersaturated solution (like described above), results in a solution that contains solute at a concentration greater than its equilibrium solubility at the lower temperature. This solution is relatively stable. However, precipitation of the excess solute can be initiated by adding a seed crystal or by mechanically agitating the solution. Some hand warmers, such as the one pictured below, take advantage of this behavior.
This hand warmer produces heat when the sodium acetate in a supersaturated solution precipitates. Precipitation of the solute is initiated by a mechanical shockwave generated when the flexible metal disk within the solution is “clicked.”

A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. These reactions are common in nature and are responsible for the formation of coral reefs in ocean waters and kidney stones in animals. They are used widely in industry for the production of a number of commodity and specialty chemicals.
The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility, defined as the maximum concentration of a substance that can be achieved under specified conditions. Substances with relatively large solubilities are said to be soluble. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Substances with relatively low solubilities are said to be insoluble, and these are the substances that readily precipitate from solution.
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “CHEMISTRY: ATOMS FIRST 2E”. ACCESS FOR FREE AT Chemistry: Atoms First 2e. LICENSE: CREATIVE COMMONS ATTRIBUTION 4.0 INTERNATIONAL