Table of Contents |
The identity of a substance is defined not only by the types of atoms or ions it contains but by the quantity of each type of atom or ion. In chemistry, the unit for the amount of a substance is the mole. The mole is an amount unit similar to familiar units like pair, dozen, gross, etc. It provides a specific measure of the number of atoms or molecules in a sample of matter. The mole provides a link between an easily measured macroscopic property—bulk mass—and an extremely important fundamental microscopic property—the number of atoms, molecules, and so forth.
A mole of a substance is that amount in which there are 6.02214076 × 1023 discrete entities (atoms or molecules). This large number is a fundamental constant known as Avogadro's number (NA) or the Avogadro constant in honor of Italian scientist Amedeo Avogadro. This constant is properly reported with an explicit unit of “per mole,” a conveniently rounded version being 6.022 × 1023 molecules (or atoms)/mol.
Consistent with its definition as an amount unit, 1 mole of any element contains the same number of atoms as 1 mole of any other element. The masses of 1 mole of different elements, however, are different, since the masses of the individual atoms are drastically different. The molar mass of an element (or compound) is the mass in grams of 1 mole of that substance, a property expressed in units of grams per mole (g/mol).

In the image above, each sample contains 6.022 ×1023 atoms —1.00 mol of atoms. From left to right (top row): 65.4 g zinc, 12.0 g carbon, 24.3 g magnesium, and 63.5 g copper. From left to right (bottom row): 32.1 g sulfur, 28.1 g silicon, 207 g lead, and 118.7 g tin.
The molar mass of any substance is numerically equivalent to its atomic or formula weight in amu. Per the amu definition, a single 12C atom weighs 12 amu (its atomic mass is 12 amu). A mole of 12C atoms weighs 12 g (its molar mass is 12 g/mol). This relationship holds for all elements, since their atomic masses are measured relative to that of the amu-reference substance, 12C. Extending this principle, the molar mass of a compound in grams is likewise numerically equivalent to its formula mass in amu.

In the image above, each sample contains 6.022 × 1023 molecules or formula units—1.00 mol of the compound or element. Clockwise from the upper left: 130.2 g of C8H17OH (1-octanol, formula mass 130.2 amu), 454.4 g of HgI2 (mercury(II) iodide, formula mass 454.4 amu), 32.0 g of CH3OH (methanol, formula mass 32.0 amu) and 256.5 g of S8 (sulfur, formula mass 256.5 amu).
| Element | Average Atomic Mass (amu) | Molar Mass (g/mol) | Atoms/Mole |
|---|---|---|---|
| C | 12.01 | 12.01 | 6.022 × 1023 |
| H | 1.008 | 1.008 | 6.022 × 1023 |
| O | 16.00 | 16.00 | 6.022 × 1023 |
| Na | 22.99 | 22.99 | 6.022 × 1023 |
| Cl | 35.45 | 35.45 | 6.022 × 1023 |
While atomic mass and molar mass are numerically equivalent, keep in mind that they are vastly different in terms of scale, as represented by the vast difference in the magnitudes of their respective units (amu versus g).

Being able to use the periodic table (see below) to calculate the molar mass of an atom or molecule is a vital skill needed to perform any of the calculations in this lesson. On the periodic table, the average atomic mass of each element is given in amu which is equivalent to g/mole. Therefore, sodium has an amu = 22.99, which is equivalent to 22.99 g/mol for the molar mass of sodium. Likewise, silicon would have a molar mass of 28.09 g/mol, and chlorine would have a molar mass of 35.45 g/mol.
IN CONTEXT
Recall that some elements form diatomic elements. Fluorine exists as F2 and oxygen as O2.
To calculate the molar mass of diatomic fluorine or oxygen, we need to multiply the molar mass by two. For example, the molar mass of fluorine (F2) = 19.00 x 2 = 38 g/mol. The molar mass of oxygen (O2) = 16.00 x 2 = 32 g/mol.
To calculate the molar mass of an element, you only need to go to the periodic table and look up the average atomic mass of the element, which is the molar mass (unless it is a diatomic and then you have to multiply the molar mass by two).
But to calculate the molar mass of a molecule, such as H2O, you need to add up the molar masses of all the elements in the molecule. Water (H2O) is composed of two atoms of H and 1 atom of O. Each H = 1.008 g/mol and each oxygen = 16.00 g/mol, so 2 H (2 x 1.008) plus one O (1 x 16.00) = 2.016 + 16.00 = 18.01 (using significant figure rules for adding). Therefore, the molar mass of water = 18.01 g/mol.
Follow this link to WebElements to view an accessible version of the periodic table of elements.

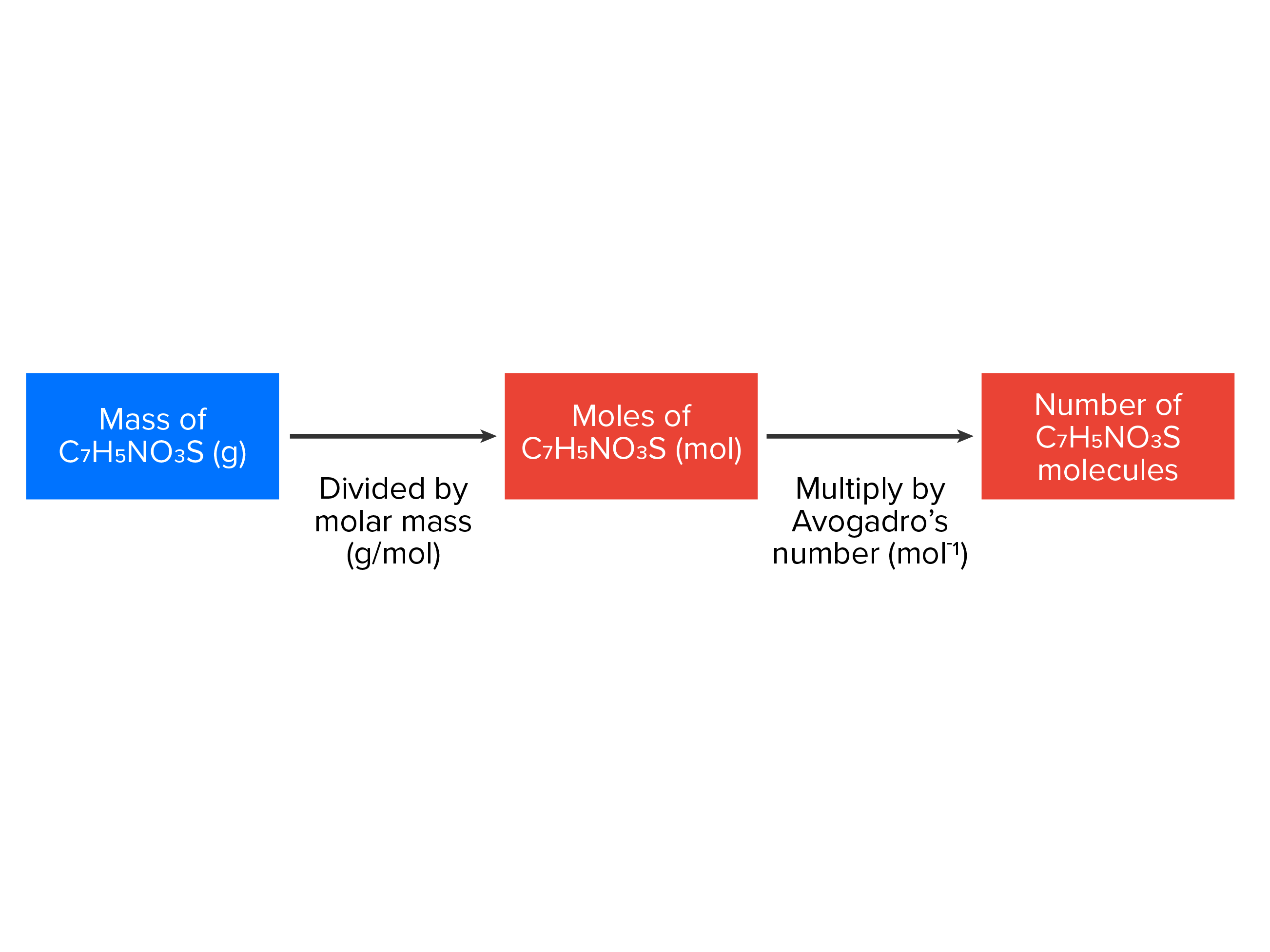
The relationships between formula mass, the mole, and Avogadro’s number can be applied to compute various quantities that describe the composition of substances and compounds. For example, if we know the mass and chemical composition of a substance, we can determine the number of moles and calculate the number of atoms or molecules in the sample. Likewise, if we know the number of moles of a substance, we can derive the number of atoms or molecules and calculate the substance’s mass. The formulas for these conversions are below.
A good way to think about conversions is to use a concept map, which is just a visual representation of the information you need to remember. The concept maps below represent the mathematical formulas you need to know to convert between grams, moles and particles (atoms or molecules).
| Formula | Concept Map |
|---|---|
| grams/molar mass = moles |

|
| moles x molar mass = grams |
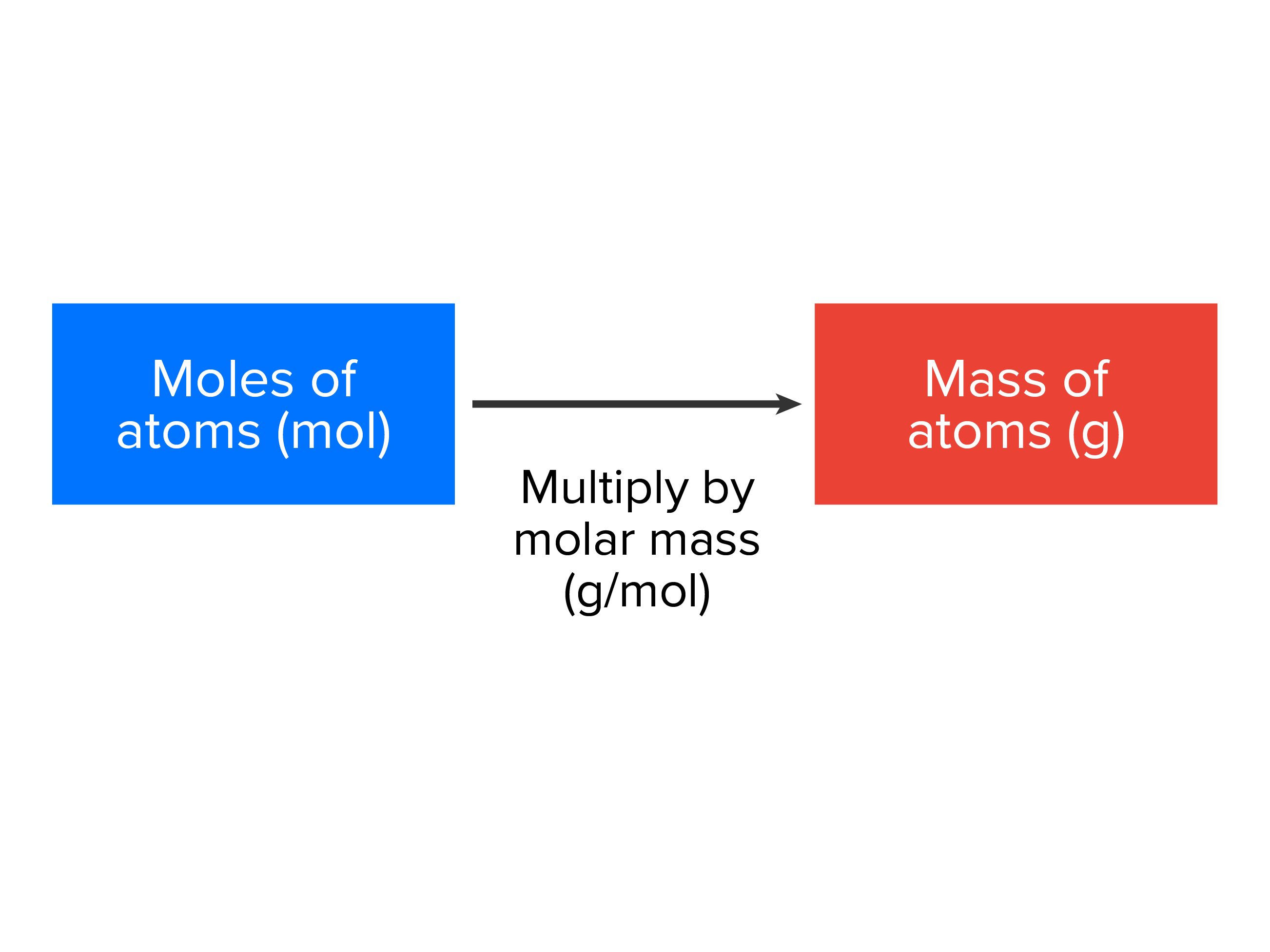
|
| moles x Avogadro’s number (6.022 x 1023) = molecules (or atoms) |

|
| molecules (or atoms)/Avogadro’s number (6.022 x 1023) = moles |
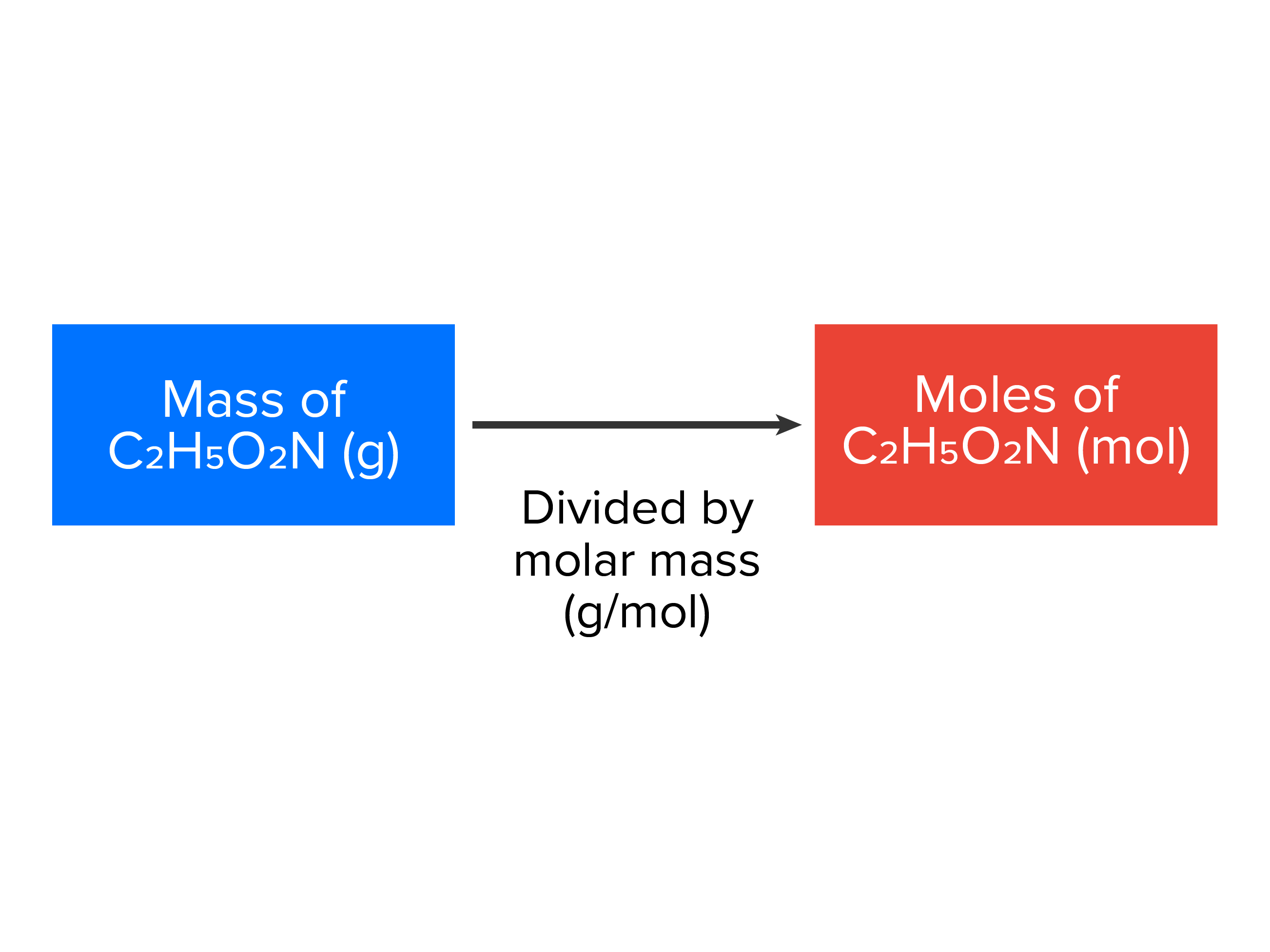
|
IN CONTEXT
While the concept of a mole might be unfamiliar to you, you do calculations similar to these all the time without any issue. If someone said they have a dozen eggs, you know right away that is 12 eggs or if someone said they have a pair of tickets to a concert, you know they have 2 tickets.
Think of moles the same way. If I tell you I have a mole of carbon, that means I have 12.01 grams of carbon or 6.022 x 1023 atoms of carbon. So, what if I said I have 3 moles of carbon, how many grams would I have? Think of this the same way as you would with dozens. If someone said they have 3 dozen donuts, you would know they have 36 donuts (3 x 12). So, if I have 3 moles of carbon, I have 36.03 grams of carbon (3 x 12.01).
What if someone said they have 48 donuts, and you were asked how many dozens of donuts they have? In your head, you would take 48/12 = 4 dozen. So if I said you have 105 grams of carbon, how many moles do you have? You should think the same way. There are 12.01 grams in a mole, so 105/12.01 = 8.74 moles.
Below are examples of using these formulas in calculations. Make sure you can do these types of calculations.
EXAMPLE
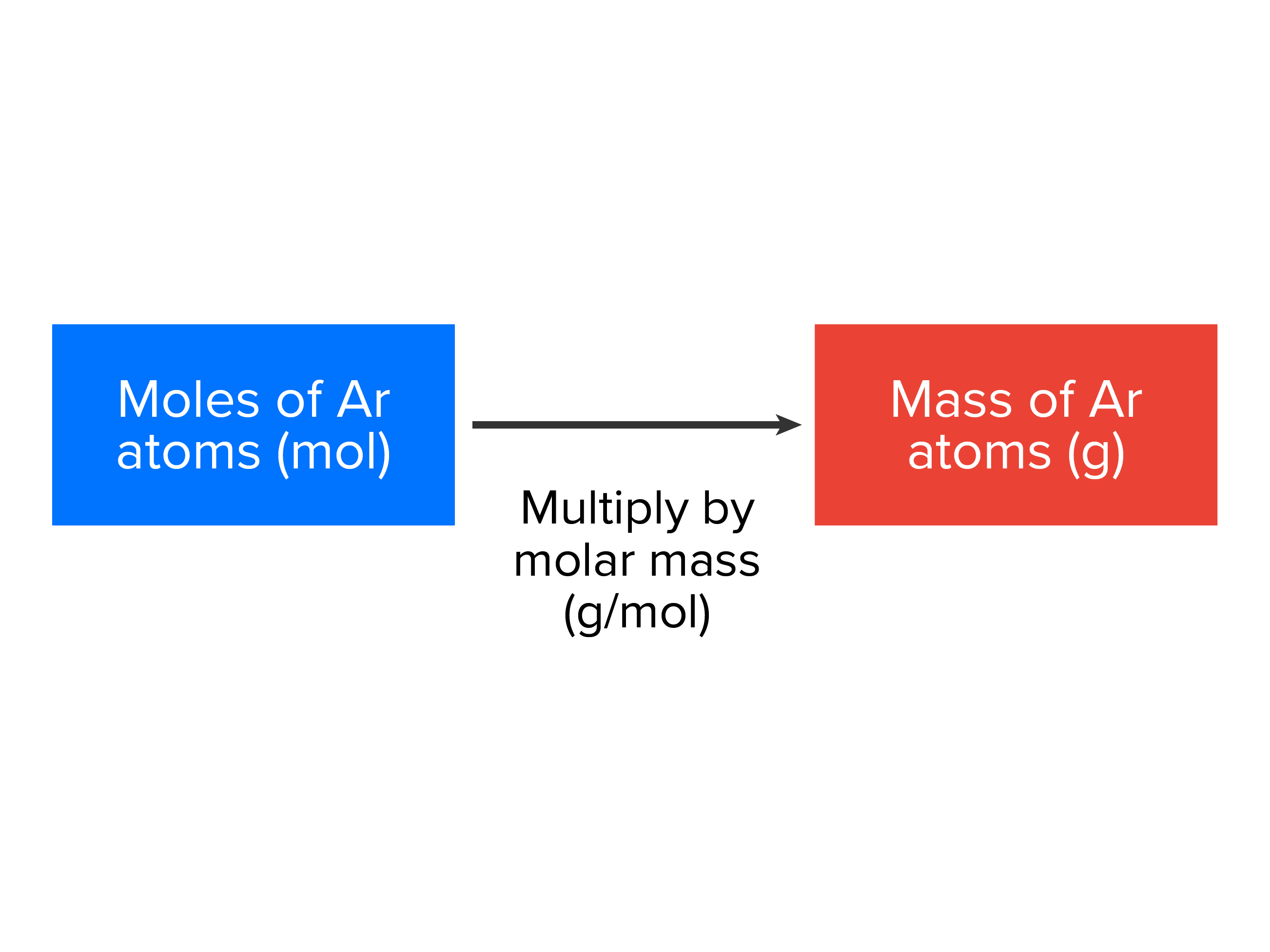
EXAMPLE
EXAMPLE
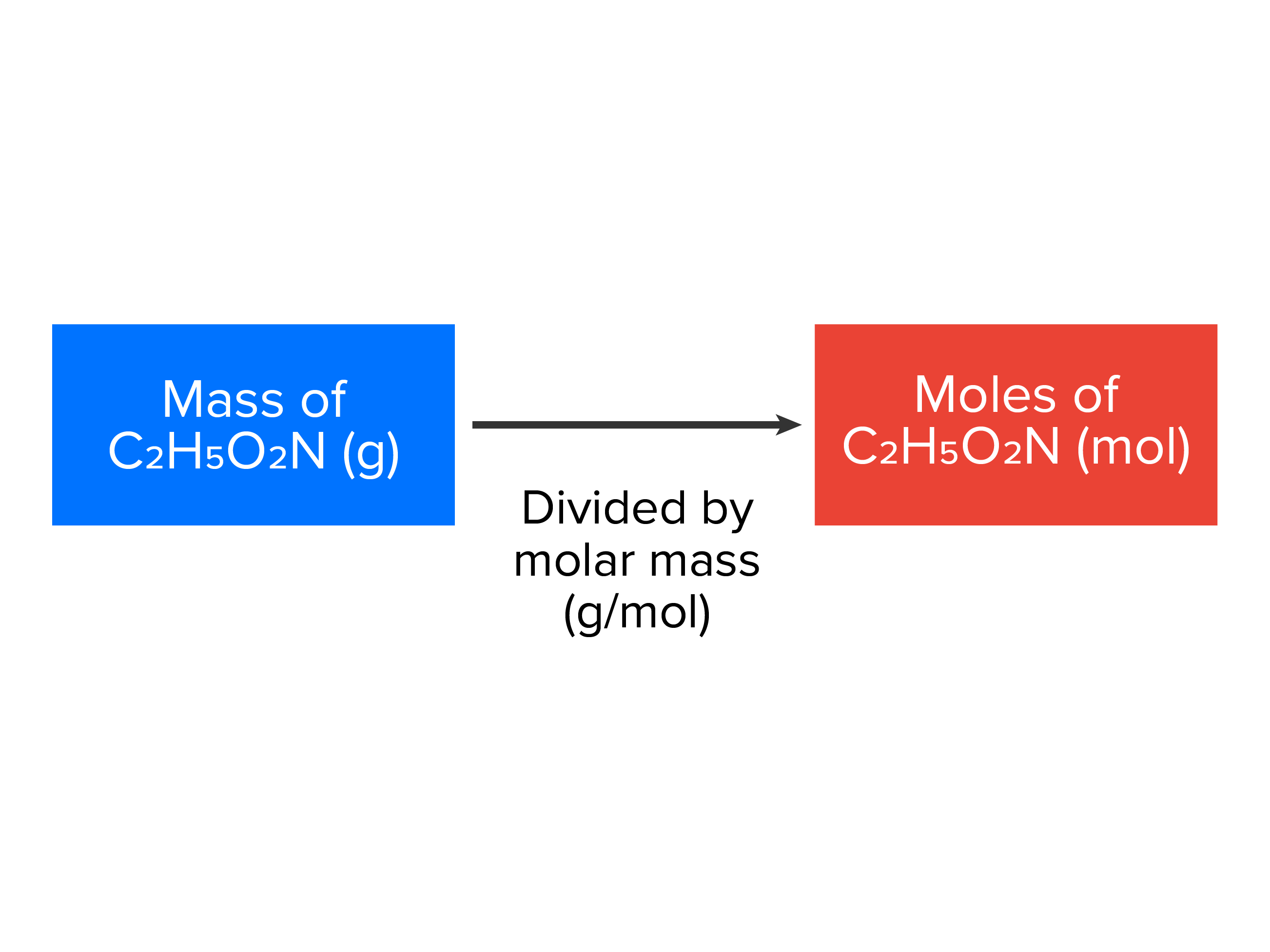
EXAMPLE

EXAMPLE
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “CHEMISTRY: ATOMS FIRST 2E”. ACCESS FOR FREE AT Chemistry: Atoms First 2e. LICENSE: CREATIVE COMMONS ATTRIBUTION 4.0 INTERNATIONAL