Table of Contents |
Much of the body is made of protein, and these proteins take on a myriad of forms. They represent cell signaling receptors, signaling molecules, structural members, enzymes, intracellular trafficking components, extracellular matrix scaffolds, ion pumps, ion channels, oxygen, and CO₂ transporters (hemoglobin).
Amid all these necessary functions, proteins also hold the potential to serve as a metabolic fuel source. Proteins are not stored for later use, so excess proteins must be converted into glucose or triglycerides, and used to supply energy or build energy reserves.

The digestion of proteins begins in the stomach. When protein-rich foods enter the stomach, they are greeted by a mixture of the enzyme pepsin and hydrochloric acid (HCl; 0.5%). The latter produces an environmental pH of 1.5–3.5 that denatures proteins within food. Pepsin cuts proteins into smaller polypeptides and their constituent amino acids. When the food–gastric juice mixture (chyme) enters the small intestine, the pancreas releases sodium bicarbonate to neutralize the HCl. This helps to protect the lining of the intestine.
As you previously learned, the small intestine also releases digestive hormones, including secretin and CCK, which stimulate digestive processes to further break down proteins. Secretin also stimulates the pancreas to release sodium bicarbonate.
The pancreas releases most of the digestive enzymes, including the proteases trypsin, chymotrypsin, and elastase, which aid protein digestion. Together, all of these enzymes break complex proteins into smaller individual amino acids, which are then transported across the intestinal mucosa to be used to create new proteins or to be converted into fats or acetyl-CoA and used in the citric acid cycle.
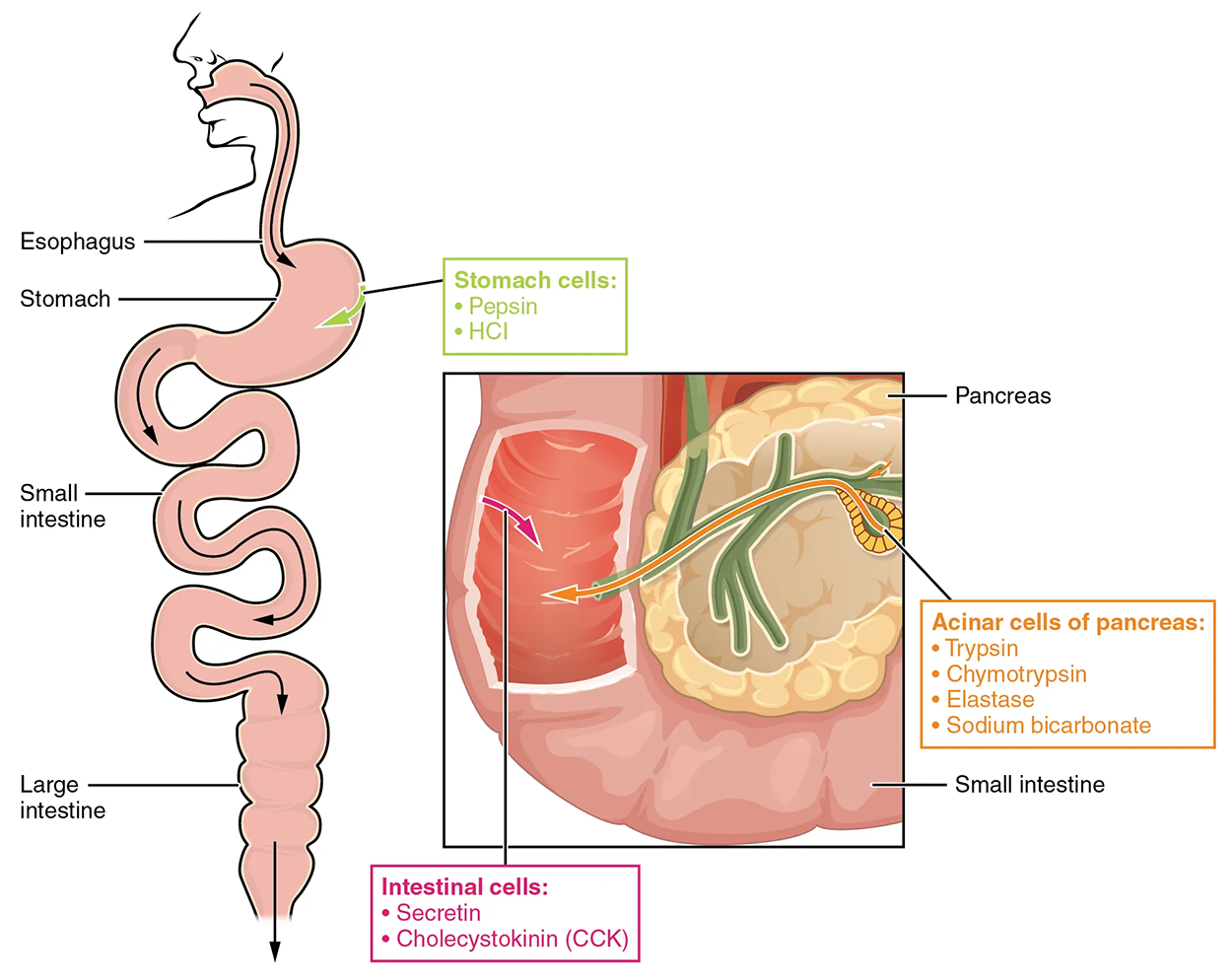
To avoid breaking down the proteins that make up the pancreas and small intestine, pancreatic enzymes are released as inactive proenzymes that are only activated in the small intestine. In the pancreas, vesicles store trypsin and chymotrypsin as trypsinogen and chymotrypsinogen. Once released into the small intestine, an enzyme found in the wall of the small intestine, called enterokinase, binds to trypsinogen and converts it into its active form, trypsin. Trypsin then binds to chymotrypsinogen to convert it into active chymotrypsin. Trypsin and chymotrypsin break down large proteins into smaller peptides during the proteolysis process (you were introduced to this process a few lessons ago).
These smaller peptides are catabolized into their constituent amino acids, which are transported across the apical surface of the intestinal mucosa in a process that is mediated by sodium–amino acid transporters. These transporters bind sodium and then bind the amino acid to transport it across the membrane. At the basal surface of the mucosal cells, the sodium and amino acid are released. The sodium can be reused in the transporter, whereas the amino acids are transferred into the bloodstream to be transported to the liver and cells throughout the body for protein synthesis.
Freely available amino acids are used to create proteins. If amino acids exist in excess, the body has no capacity or mechanism for their storage; thus, they are converted into glucose or ketones, or they are decomposed. Amino acid decomposition results in hydrocarbons and nitrogenous waste. However, high concentrations of nitrogenous byproducts are toxic. Consequently, the urea cycle processes nitrogen and facilitates its excretion from the body.
| Term | Pronunciation | Audio File |
|---|---|---|
| Secretin | se·cre·tin |
|
| Chymotrypsin | chy·mo·tryp·sin |
|
| Trypsinogen | tryp·sin·o·gen |
|
| Chymotrypsinogen | chy·mo·tryp·sin·o·gen |
|
The urea cycle is a set of biochemical reactions that produces urea from ammonium ions to prevent a toxic level of ammonium in the body. It occurs primarily in the liver and, to a lesser extent, in the kidney. Prior to the urea cycle, ammonium ions are produced from the breakdown of amino acids, which also occurs in the liver. In these reactions, an amine group, or ammonium ion, from the amino acid is exchanged with a keto group on another molecule. This transamination event, in which an amine group is transferred from one molecule to another to turn nitrogen waste into ammonia so that it can enter the urea cycle, creates a molecule that is necessary for the citric acid cycle and an ammonium ion that enters into the urea cycle to be eliminated.
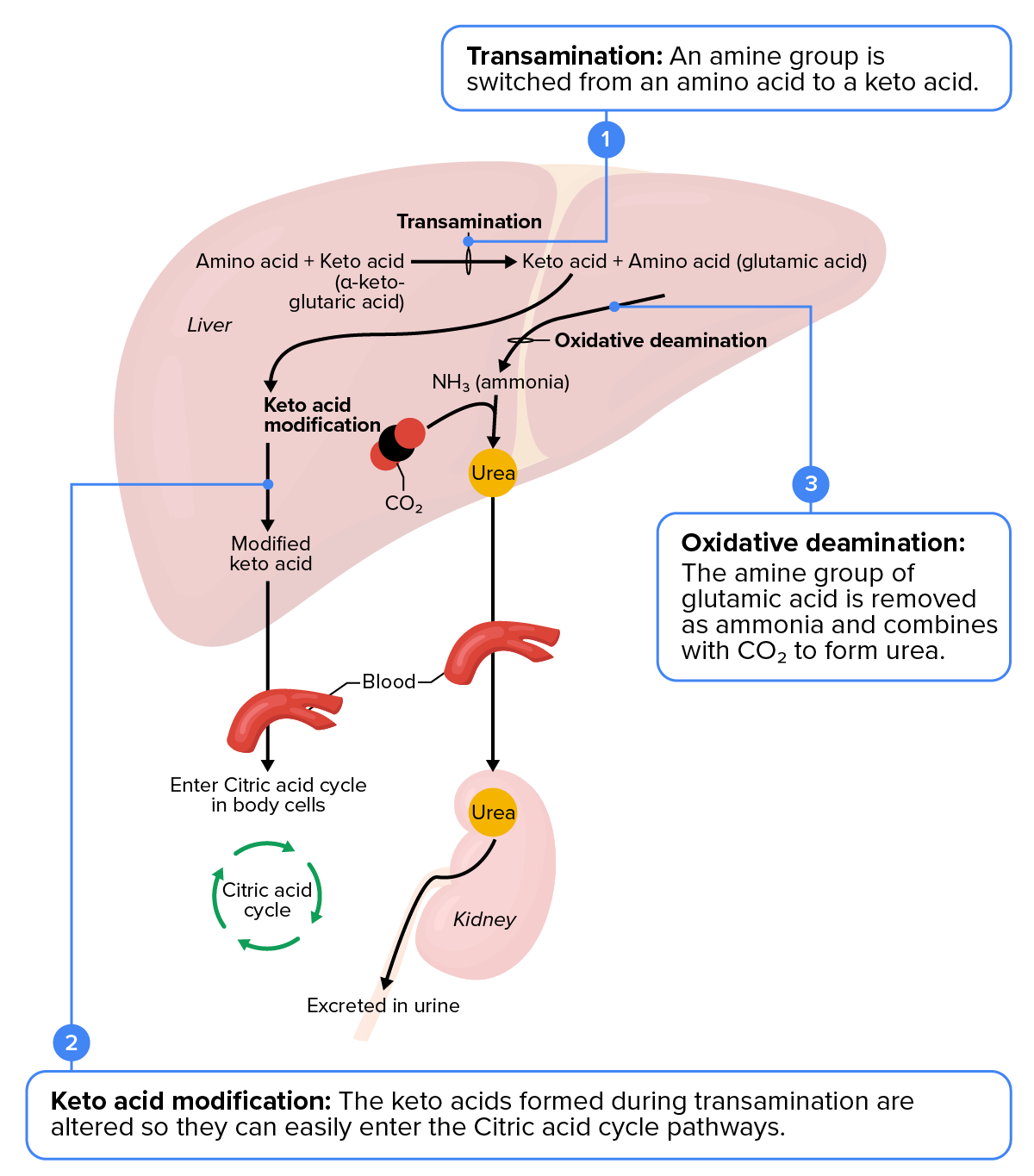
In the urea cycle, ammonium is combined with CO₂, which results in urea and water. The urea is eliminated through the kidneys in the urine.
Amino acids can also be used as a source of energy, especially in times of starvation. Because the processing of amino acids results in the creation of metabolic intermediates, such as pyruvate, acetyl-CoA, oxaloacetate, and α-ketoglutarate, amino acids can serve as a source of energy production through the citric acid cycle (see the top image below). The bottom image below summarizes the pathways of catabolism and anabolism for carbohydrates, lipids, and proteins.
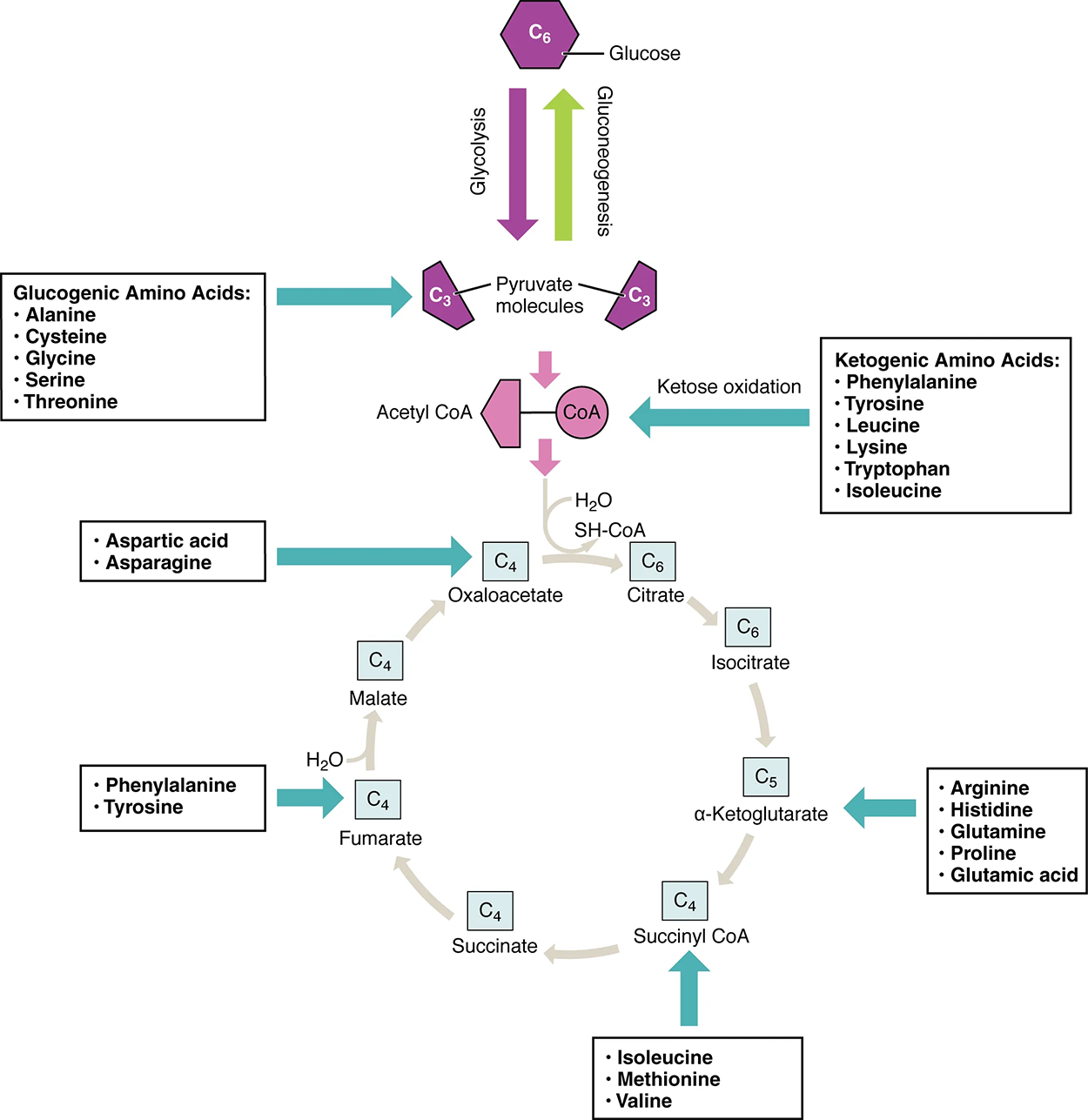
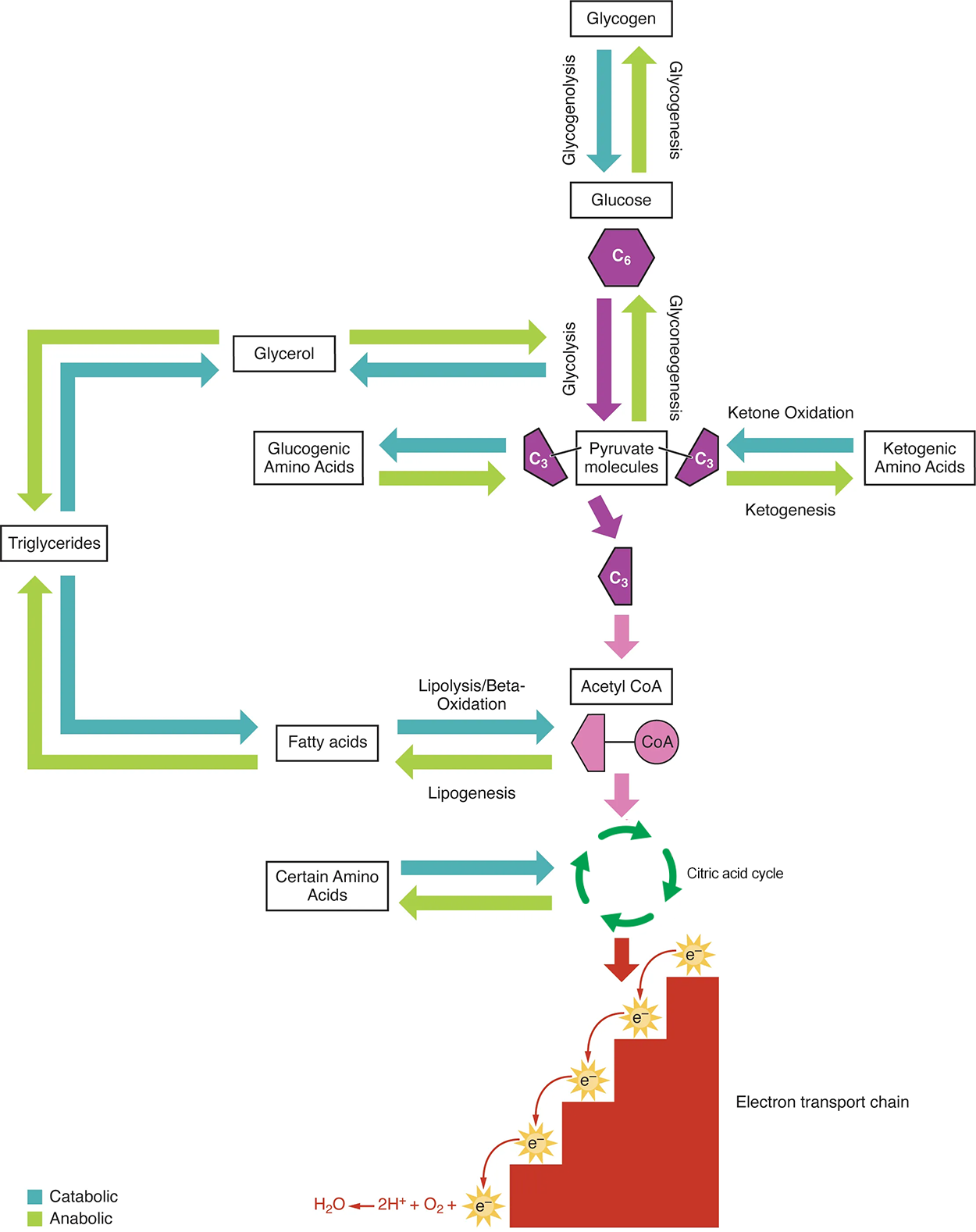
EXAMPLE
| Term | Pronunciation | Audio File |
|---|---|---|
| Transamination | trans·am·i·na·tion |
|
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX "ANATOMY AND PHYSIOLOGY 2E" ACCESS FOR FREE AT OPENSTAX.ORG/DETAILS/BOOKS/ANATOMY-AND-PHYSIOLOGY-2E. LICENSE: CREATIVE COMMONS ATTRIBUTION 4.0 INTERNATIONAL