Table of Contents |
Liquid water is essential to life on our planet, and chemistry involving the characteristic ions of water, H+ and OH–, is widely encountered in nature and society. Acid-base chemistry involves the transfer of hydrogen ions from donors (acids) to acceptors (bases). These H+ transfer reactions are reversible, and the equilibria established by acid-base systems are essential aspects of many parts of our daily lives.
There are many acids that are part of our daily lives. Vinegar is dilute acetic acid (CH3COOH). Vitamin C (ascorbic acid) is essential to our diet. Hydrochloric acid (HCl) is the main constituent of our stomach acid. Amino acids are found in all proteins and they are compounds that contain both an acid and a base in their structure.
Sulfuric acid (H2SO4) is the “battery acid” found in lead car batteries. Sulfuric acid is also used industrially in the manufacturing of fertilizer, synthetic clothing fibers, metal procession, paints, pulp, paper, and many other useful products. Sulfuric acid is the number one produced chemical in the world. (Phosphoric acid is the eighth most produced chemical in the world).

Carbonic acid (H2CO3) and phosphoric acid (H3PO4) are found in many soft drinks. Tartaric acid is found in wine. Acetic, pyruvic, lactic, malic, and citric acid are found in beer. Lactic acid is also found in milk. Fruit juices will contain malic and citric acid. Tea contains succinic, oxalic, malic, and citric acid. Coffee contains citric, acetic, lactic, malic, and phosphoric acid.
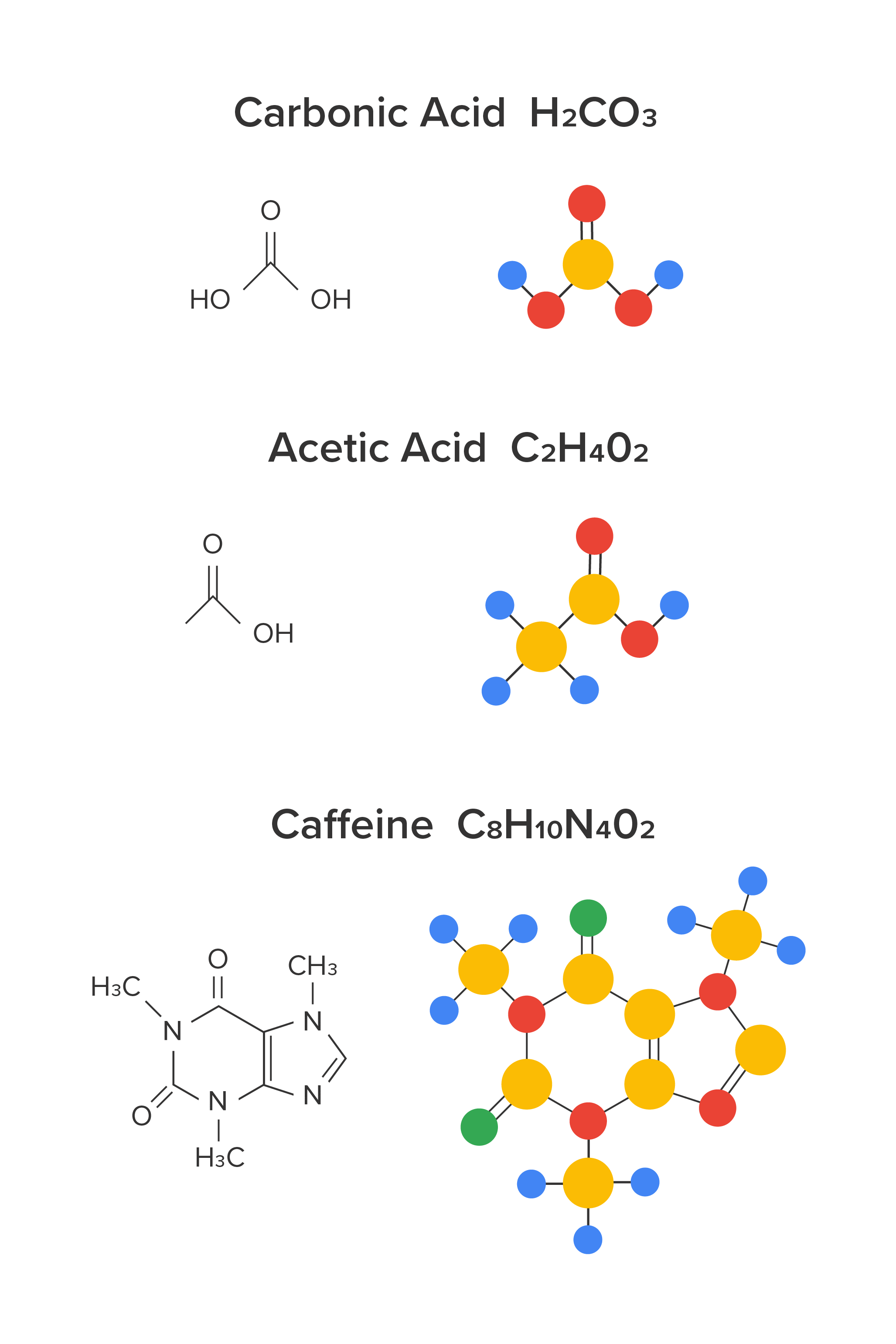
Lactic acid is generated in our bodies during exercise and can cause lactic acidosis, a condition where your blood becomes too acidic. Citric acid is a main metabolite of the citric acid cycle, which our bodies use to convert nutrients (sugar, fats, and proteins) into energy. Citric acid is also found in citrus fruit such as lemons, limes, and oranges.
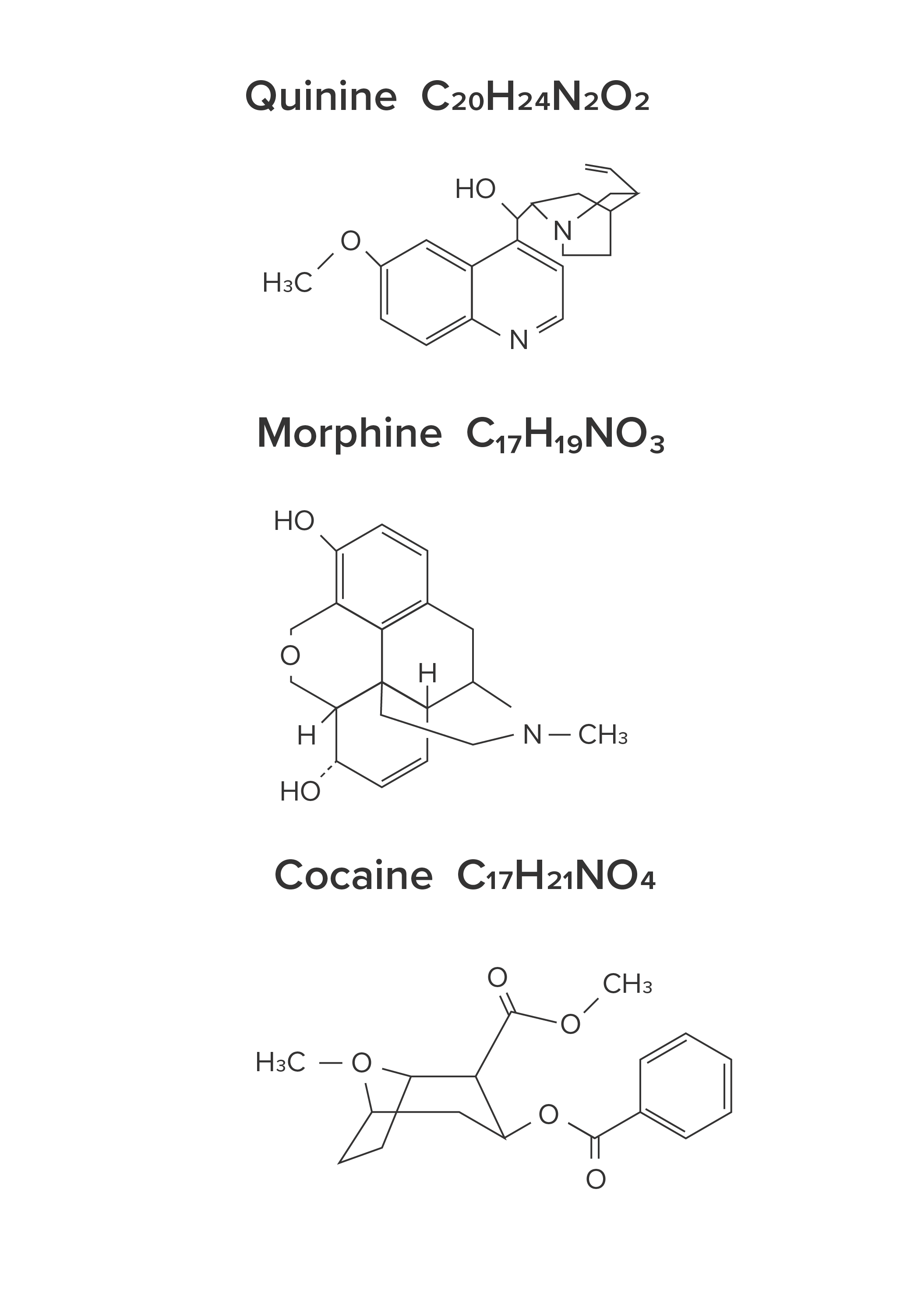
There are many bases that are also part of our daily lives. As mentioned above, proteins contain amino acids which contain both an acid and a base as part of their structure. Alkaloids are a class of organic compounds derived from plants that contain nitrogen atoms. Nitrogen-containing organic compounds are often basic. Alkaloids in our daily lives include caffeine (which is found in many beverages such as coffee, tea, and soft drinks) and nicotine (which is found in tobacco).
Other alkaloids include quinine (found in tonic water), morphine (an analgesic), cocaine (an anesthetic), and many others, including codeine, scopolamine, atropine, strychnine, methamphetamine, mescaline, and ephedrine. Analgesics are chemicals that are used as pain relievers. Anesthetics are chemicals that are used to temporarily prevent someone from feeling pain.
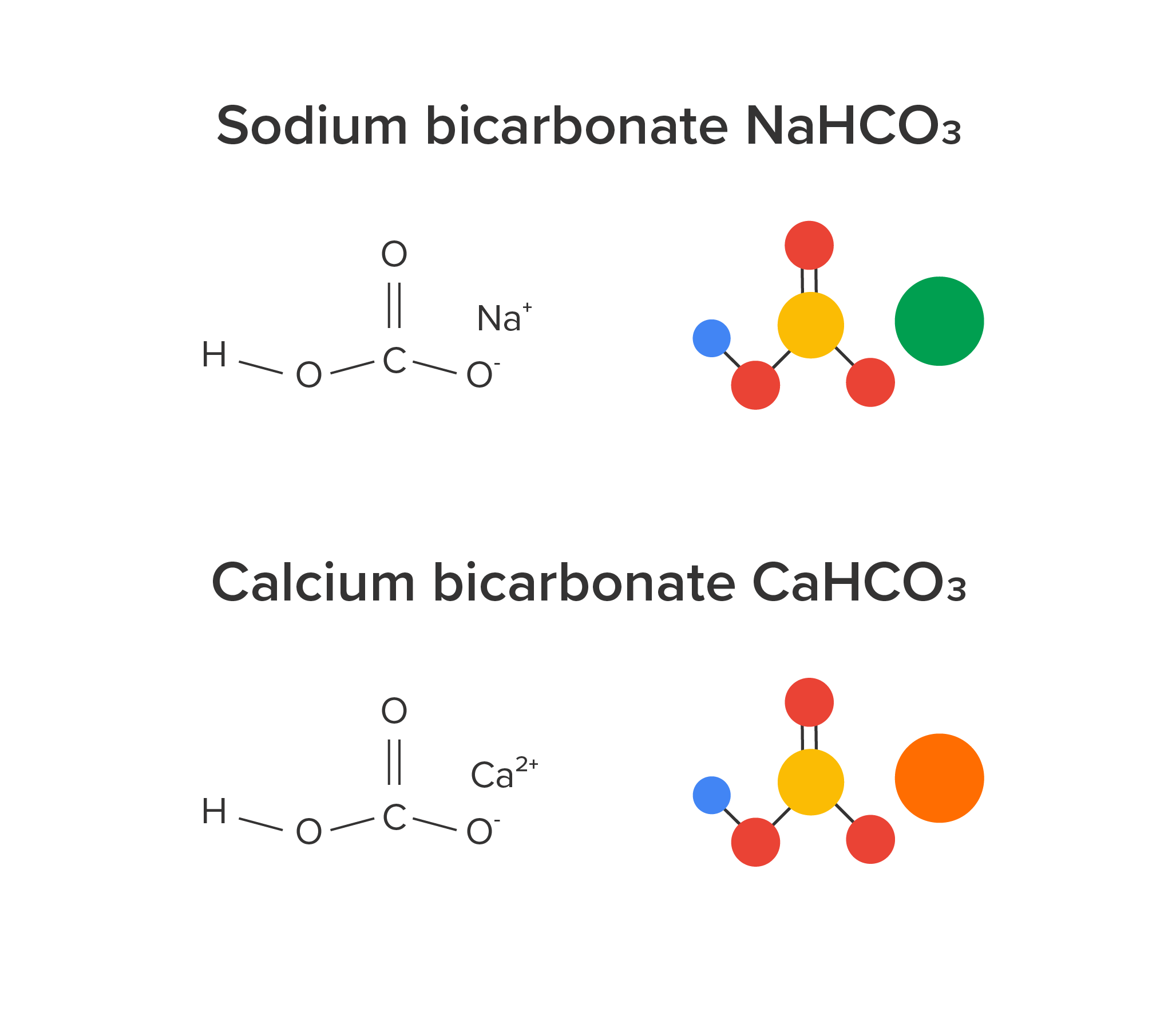
Another type of base is antacids, which are chemicals that counteract the effects of acids in your stomach. This process is also called neutralization, which is a reaction of an acid and base to form salt and water. A salt is an ionic compound formed from the cation of a base and the anion of an acid.
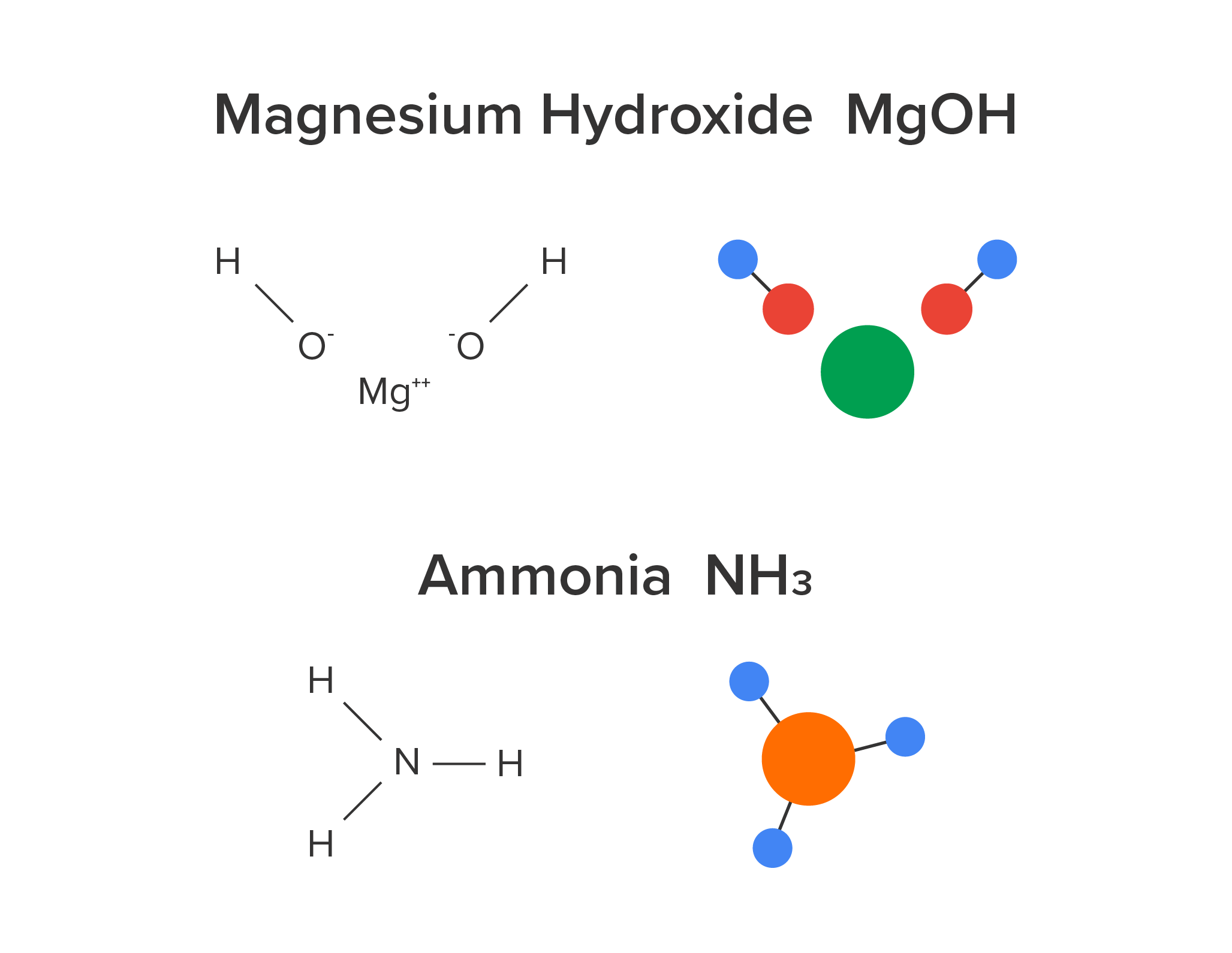
Common antacids include sodium bicarbonate (NaHCO3, baking soda), calcium carbonate (CaCO3, Alka-Seltzer and Tums), and magnesium hydroxide (Mg(OH)2, Milk of Magnesia). Rolaids are another antacid, which is made of calcium carbonate and magnesium hydroxide.
Other bases in our daily lives include soap, oven cleaner, drain cleaner, toothpaste, bleach, ammonia, lime (Ca(OH)2), and cement. Many household cleaners are also bases.
Many bases are used in industrial processes. These common bases include potassium hydroxide (KOH), sodium hydroxide or lye (NaOH), ammonia (NH3), magnesium hydroxide (Mg(OH)2), and borax.
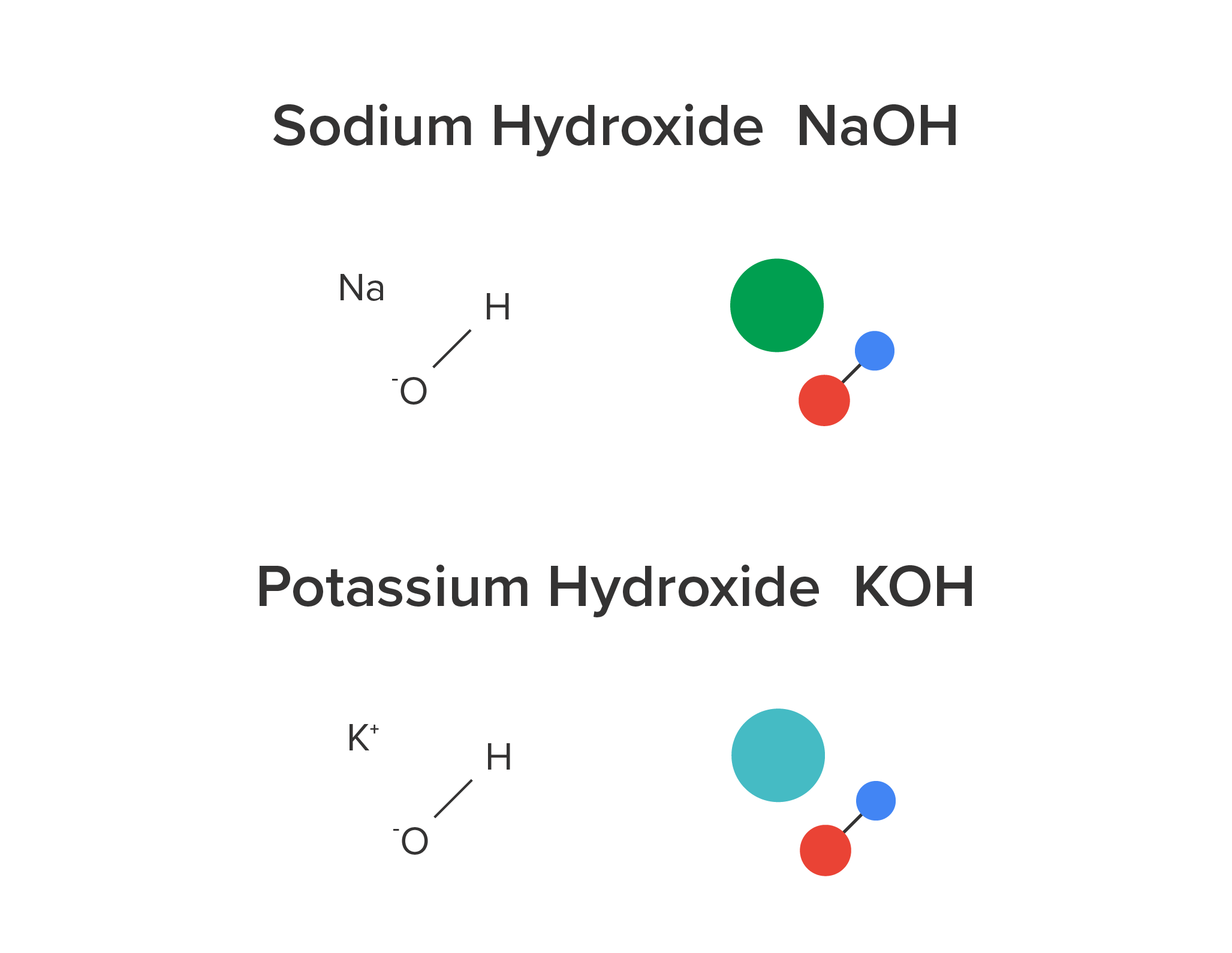
Sodium hydroxide is used to manufacture soap and paper. Calcium hydroxide is used for multiple processes including food preservation, cement production, and sewage treatment. Potassium hydroxide is used in batteries, liquid soaps, and etching semiconductors. Ammonia is used in the manufacturing of fertilizers. Magnesium hydroxide is used for the insulation of wires, and as a flame retardant additive. Borax is used for water softening, welding, and gold mining.
As previously mentioned, acids are compounds that donate hydrogen ions. The term acid comes from the Latin word acidus, which means “sour or tart.” Other acids also come from Latin roots. Acetic acid comes from the Latin word acetum, which means “vinegar.” Formic acid comes from the Latin word formica, which means “ant. ”
Most acids share the same common properties. Most acids have a sour taste, burn to the touch, change litmus from blue to red, react with metals to produce hydrogen (H2) gas, react with hydroxide bases to produce water and salt, and react with carbonates to produce carbon dioxide.
Recall that fruits such as lemons and lime contain citric acid, and they have a very sour taste. Citric acid will also burn you if you have an open wound. Imagine eating a piece of citrus fruit with a cut on your lip and the burning you will feel. Citric acid is not strong enough to burn your skin directly, but nitric acid will cause chemical burns to the skin, specifically, turning the skin yellow. Hydrochloric and sulfuric acid can cause actual burns to the skin as they destroy the tissues.
Another acid that is extremely dangerous is hydrofluoric acid (HF). While HF is a very weak acid and does not burn the skin at all, it is one of the most dangerous chemicals and a chemical you should not deal with at all. A small amount (about the size of the palm) that comes in contact with the skin can be fatal. HF is such a weak acid that you will not feel the pain of the HF in contact with your skin. As it absorbs in your body, however, it will sequester magnesium and calcium, which are vital to such organs as the heart, liver, and kidney.
As seen previously, bases are compounds that accept hydrogen ions. Most bases share the same common properties. Most bases have a bitter or caustic taste, are slippery or soapy to the touch, change litmus from red to blue, and react with acids.
The bitter or caustic taste of bases can be seen in the caffeine in coffee or tea. You can also taste the bitterness in soap. You can even taste the bitterness if you taste some novocaine from your dentist when they anesthetize your mouth for procedures. When sodium hydroxide or other strong bases touch your skin, they will react with the fatty acids and oils on the surface of your skin and generate a molecule that is in essence a soap molecule, so you can feel that slipperiness from the soap molecule that is made in the reaction of a base with your oils and acids on your skin.
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “CHEMISTRY: ATOMS FIRST 2E”. ACCESS FOR FREE AT Chemistry: Atoms First 2e. LICENSE: CREATIVE COMMONS ATTRIBUTION 4.0 INTERNATIONAL