Table of Contents |
Ozone is a colorless and tasteless gas. However, unlike oxygen, which is odorless, colorless, and tasteless, ozone has an odor to it. Sometimes the odor is described as a pungent chlorine-like smell. It also can be described as the smell that accompanies an electrical discharge. Unlike oxygen, ozone is not involved in combustion. Ozone is also a bactericide (kills bacteria), decolorizer (removes color from water), and deodorizer (removes odor from water). These properties make ozone very useful in water treatment. Ozone is used in swimming pools, hot tubs, and even bottled water.
Ozone has the molecular formula O . Ozone is formed from oxygen. The formation of ozone requires energy. This energy can be in the form of lightning or sunlight. Ozone is a very toxic chemical and is considered a pollutant near the surface of the Earth. However, stratospheric ozone is necessary to protect us from dangerous ultraviolet radiation.
. Ozone is formed from oxygen. The formation of ozone requires energy. This energy can be in the form of lightning or sunlight. Ozone is a very toxic chemical and is considered a pollutant near the surface of the Earth. However, stratospheric ozone is necessary to protect us from dangerous ultraviolet radiation.
EXAMPLE
The uses of ozone depend on its reactivity with other substances. It can be used as a bleaching agent for oils, waxes, fabrics, and starch, by oxidizing the colored compounds in these substances to colorless compounds. Thus, it is an alternative to chlorine as a disinfectant for water.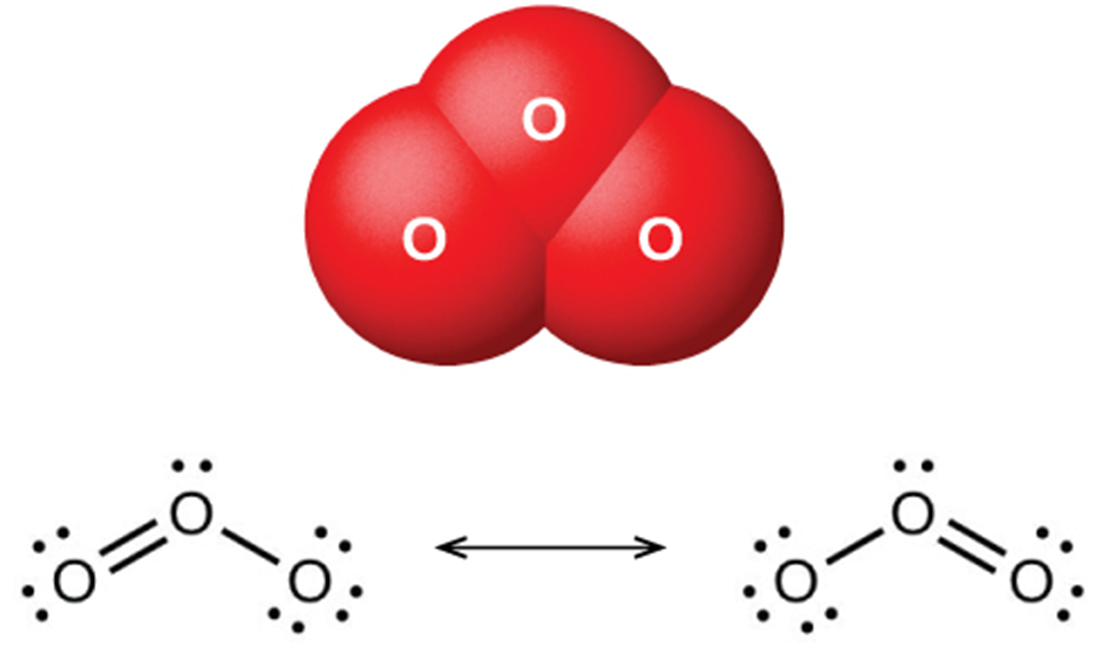
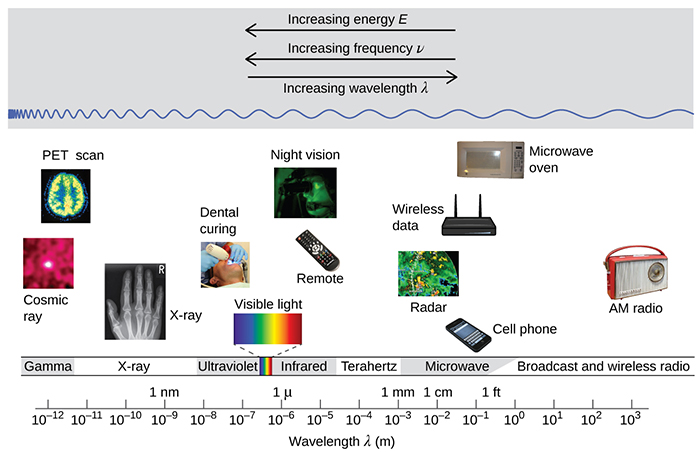
Most people only think about visible light when they think of sunlight. But sunlight consists of all forms of light or electromagnetic radiation (see the image above). The majority of sunlight is visible light, ultraviolet (UV) light, and infrared (IR) light, but there are also cosmic rays, x-rays, microwaves, and other various forms of electromagnetic radiation. However, we will focus on the main three forms of sunlight (UV, IR, and visible light).
Visible light is the form of sunlight that humans can see. It includes red, orange, yellow, green, blue, and violet light. While humans can only see this small sliver of light, other animals can see more of the electromagnetic spectrum. Pit vipers can “see” or detect infrared radiation using the pits on their faces. Reindeer, butterflies, bumblebees, and hawks can see or detect ultraviolet radiation.
When we see visible light we are actually seeing the reflection of light off a surface. For example, if you see someone wearing a blue shirt (or perceive it as blue), the shirt is absorbing all colors of light except blue, which is being reflected off of the shirt so you can perceive the shirt as blue. An orange shirt absorbs all colors except orange. A white shirt absorbs no colors and reflects all, a mixture of all forms of visible light is perceived as white light. When you see black, all colors are being absorbed and nothing is being reflected.
IN CONTEXT
Infrared light is the form of sunlight that provides warmth to the Earth. If you go outside and feel the heat of the sun, you are feeling the effects of infrared light. Infrared light falls just below red on the electromagnetic spectrum (in terms of energy or frequency). So, infrared light is actually weaker than red light. Infrared light is used to warm the atmosphere. You will learn much more about infrared light in the next lesson on climate change.
The final main form of sunlight is ultraviolet radiation. Ultraviolet is just above violet on the electromagnetic spectrum (in terms of energy or frequency). So, UV is actually stronger than violet light. UV light is strong enough to break covalent bonds, which can result in chemical changes in atoms and molecules.
UV light is a dangerous form of electromagnetic radiation that has a higher frequency than visible light. UV light and all other forms of light with higher frequencies than UV light (x-rays, gamma rays, and cosmic rays) are dangerous and damaging forms of electromagnetic radiation. Our atmosphere filters out almost all of the very deadly forms of radiation, but UV light is one dangerous form of electromagnetic radiation that can get to the surface of the Earth, where it can cause a lot of damage.
UV light is the form of light with wavelengths from 100 to 400 nm. As a note, the visible spectrum of light starts at 400 nm (violet light) and ends at 700 nm (red light). We classify UV light into three bands, UV-A, UV-B, and UV-C.
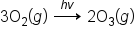
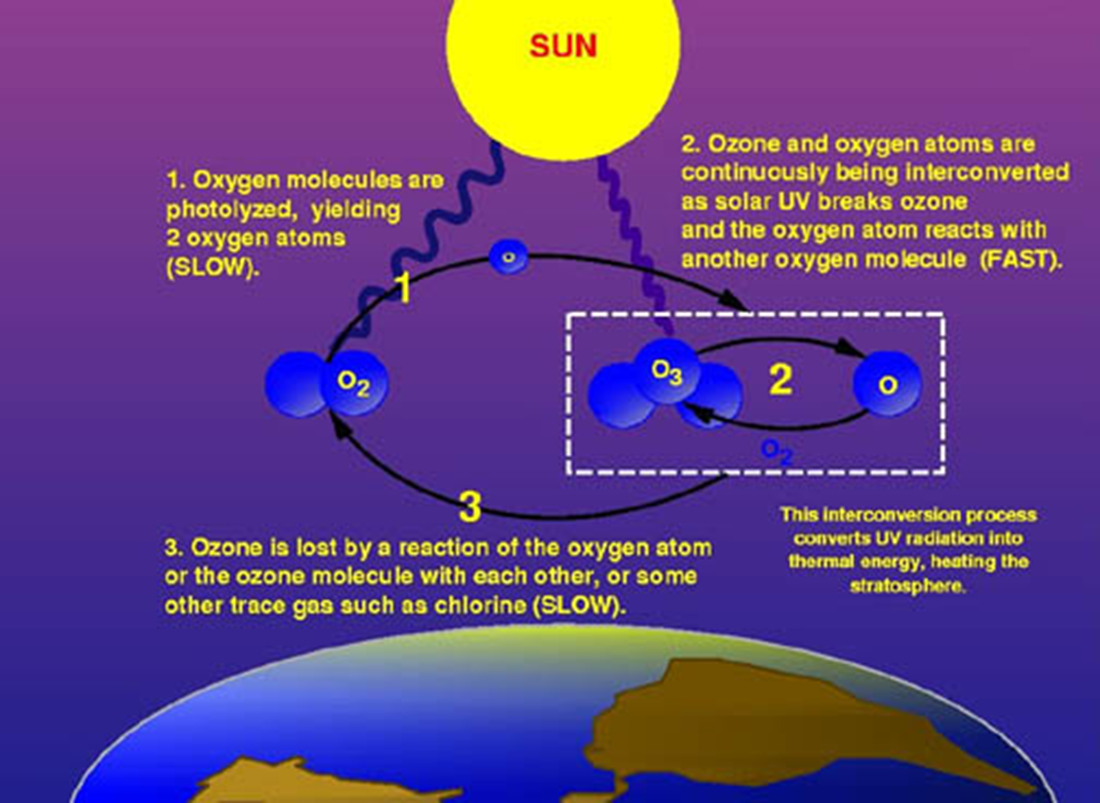
The covalent bond in ozone will absorb UV light from around 200 nm to 340 nm before the covalent bond in ozone will break. When the bond breaks in ozone, it converts the ozone back to oxygen.
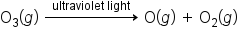
Overall, oxygen absorbs dangerous UV-C radiation from 100 to 240 nm and forms ozone in the process. Ozone absorbs dangerous UV-C and UV-B radiation from 200 to 340 nm reforming oxygen in the process. It has been determined that up to 300 million tons of ozone is created and destroyed in this process each day. As oxygen converts to ozone and back to oxygen, almost all the dangerous UV light (UV-C and UV-B) is absorbed and destroyed in the process. This process protects us from the dangerous forms of UV radiation.
The picture below summarizes the ozone cycle. In a slow process, UV light from the sun breaks apart (photolyzed) an oxygen atom, which then is rapidly converted to ozone, which rapidly decomposes via UV light to form oxygen. This interconversion between ozone and oxygen is a rapidly occurring reaction that transforms UV light from the sun into thermal energy, which heats up the stratosphere. Finally, in a slow process, the ozone reacts with chlorine or some other atom.
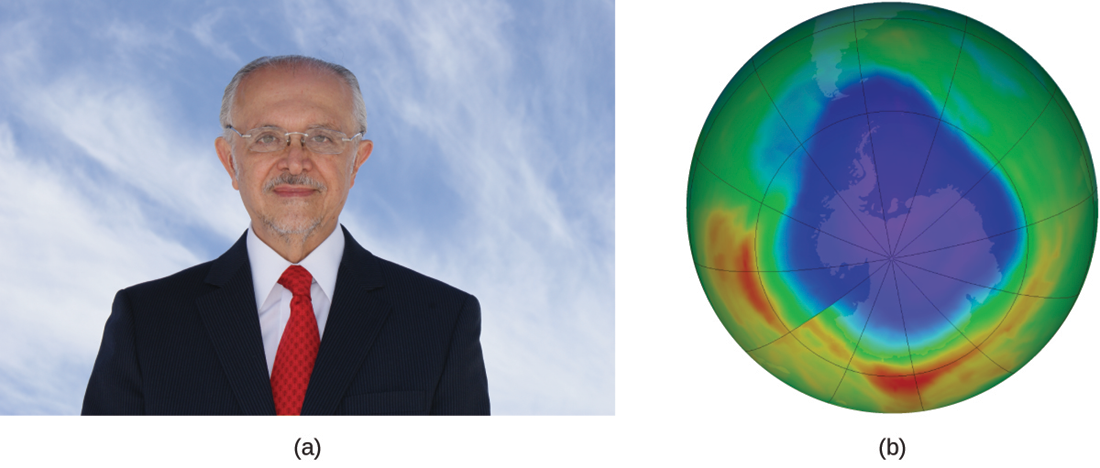
In 1974, Molina and Rowland published a paper in the journal Nature detailing the threat of chlorofluorocarbon gases to the stability of the ozone layer in the Earth’s upper atmosphere. The ozone layer protects the Earth from solar radiation by absorbing ultraviolet light. As chemical reactions deplete the amount of ozone in the upper atmosphere, a measurable “hole” forms above Antarctica, and an increase in the amount of solar ultraviolet radiation—strongly linked to the prevalence of skin cancers—reaches the Earth’s surface. The work of Molina and Rowland was instrumental in the adoption of the Montreal Protocol, an international treaty signed in 1987 that successfully began phasing out the production of chemicals linked to ozone destruction.
IN CONTEXT
Molina and Rowland demonstrated that chlorine atoms from human-made chemicals can catalyze ozone destruction in a process similar to that by which NO accelerates the depletion of ozone.
Chlorine atoms are generated when chlorocarbons or chlorofluorocarbons—once widely used as refrigerants and propellants—are photochemically decomposed by ultraviolet light or react with hydroxyl radicals. A sample mechanism is shown here using methyl chloride.
Chlorine radicals break down ozone and are regenerated by the following catalytic cycle:
A single monatomic chlorine can break down thousands of ozone molecules. Luckily, the majority of atmospheric chlorine exists as the catalytically inactive forms Cland ClONO
.
After receiving his portion of the Nobel Prize, Molina continued his work in atmospheric chemistry at MIT, until his death in 2020.

The reactive oxygen atoms recombine with molecular oxygen to complete the ozone cycle. The presence of stratospheric ozone decreases the frequency of skin cancer and other damaging effects of ultraviolet radiation. It has been clearly demonstrated that chlorofluorocarbons, CFCs (known commercially as Freons), which were present as aerosol propellants in spray cans and as refrigerants, caused depletion of ozone in the stratosphere. This occurred because ultraviolet light also causes CFCs to decompose, producing atomic chlorine. The chlorine atoms react with ozone molecules, resulting in a net removal of O molecules from the stratosphere.
molecules from the stratosphere.
There is a worldwide effort to reduce the amount of CFCs used commercially, and the ozone hole is already beginning to decrease in size as atmospheric concentrations of atomic chlorine decrease.
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “CHEMISTRY: ATOMS FIRST 2E”. ACCESS FOR FREE AT Chemistry: Atoms First 2e. LICENSE: CREATIVE COMMONS ATTRIBUTION 4.0 INTERNATIONAL