Table of Contents |
An important quality of a useful medication is that it is able to harm the pathogen without excessively harming the host (called selective toxicity). Some medications are safer than others because there is lesser risk of negative effects on the host. Bacterial cells are prokaryotic and human cells are eukaryotic; therefore, there are currently more antimicrobials available for the treatment of bacterial infections than there are for other types of infections. This is because of the differences in these cell types that provide targets for medications. Developing antiviral medications poses particular challenges because viruses force host cells to perform functions for them and targets unique to the virus (such as viral enzymes) must be identified as potential targets for drug therapies.
The safest medications have a relatively large difference between the therapeutic dose (the dose needed for effective treatment) and the dose at which the medication causes serious harm (Encyclopedia Britannica, 2019). The ratio of the therapeutic dose and the toxic dose is called the therapeutic index (Encyclopedia Britannica, 2019). There are limitations with using therapeutic indices (e.g., they are often determined using animal studies that may not exactly mimic effects in humans), but the basic concept of balancing treatment effects and adverse effects is an important consideration in choosing a medication.
Antimicrobial medications are classified based on their mode of action, which means the specific way in which the drug affects the pathogen (i.e., how the drug works). As you will learn in this lesson, modes of action vary by type of pathogen, and the widest range exists among medications that target bacteria (prokaryotic cells).
At present, there is a wider range of antimicrobial medications available to treat bacterial infections than there is for other types of infections. The image below shows major mechanisms of action of classes of antimicrobial drugs, and you will learn more about these and other mechanisms of action later in the lesson. The image shows examples of medications with the following bacterial targets: cell wall synthesis, membrane function, protein synthesis by ribosomes, metabolic pathways, and nucleic acid synthesis.
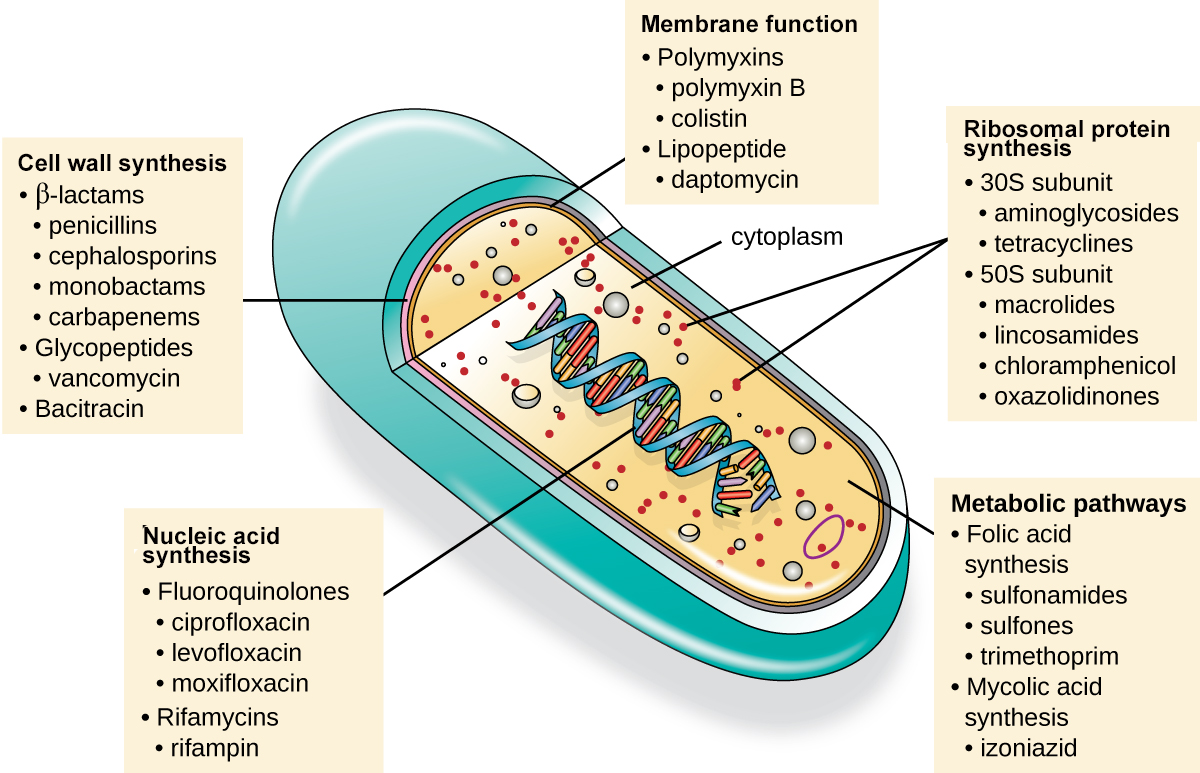
The table below describes these and additional modes of action in more detail. The next sections of the lesson describe these mechanisms of action more fully. For each section, several major examples will be described to illustrate the mechanism of action.
| Common Antibacterial Drugs by Mode of Action | ||
|---|---|---|
| Mode of Action | Target | Drug Class |
| Inhibit cell wall biosynthesis | Penicillin-binding proteins | β-lactams: penicillins, cephalosporins, monobactams, carbapenems |
| Peptidoglycan subunits | Glycopeptides | |
| Peptidoglycan subunit transport | Bacitracin | |
| Inhibit biosynthesis of proteins | 30S ribosomal subunit | Aminoglycosides, tetracyclines |
| 50S ribosomal subunit | Macrolides, lincosamides, chloramphenicol, oxazolidinones | |
| Disrupt membranes | Lipopolysaccharide, inner and outer membranes | Polymyxin B, colistin, daptomycin |
| Inhibit nucleic acid synthesis | RNA | Rifamycin |
| DNA | Fluoroquinolones | |
| Antimetabolites | Folic acid synthesis enzyme | Sulfonamides, trimethoprim |
| Mycolic acid synthesis enzyme | Isonicotinic acid hydrazide | |
| Mycobacterial adenosine triphosphate (ATP) synthase inhibitor | Mycobacterial ATP synthase | Diarylquinoline |
Bacterial cells are unique in that most contain peptidoglycan, making this an important target for antimicrobial drugs. Several classes of antibacterial medications block steps in the synthesis of peptidoglycan. These medications are effective against bacterial cells that are actively building cell walls because they stop synthesis. As a result, the bacterial cells are vulnerable to lysis as the solute concentration inside the cell is higher than in the surrounding fluid in organisms such as humans. These medications are bactericidal against susceptible microbes.
β-Lactams are an important class of antibiotics with this mode of action and this group includes the first antibiotic discovered (penicillin). These drugs are characterized by the presence of a β-lactam ring in the central structure of the molecule, as shown in several examples of these drugs in the image below. Because the drug structure is similar to that of the peptidoglycan subunit component recognized by penicillin-binding protein (PBP), which forms crosslinks of peptidoglycan, these medications inhibit peptidoglycan synthesis. Over time, new varieties of these medications have been developed, including semisynthetic varieties that have beneficial characteristics such as increased potency, expanded spectrum of activity, and longer half-lives. New versions have been developed that have structural features that help to prevent destruction of the β-lactam ring, which is sometimes destroyed by bacteria as a way of resisting harm from these drugs.
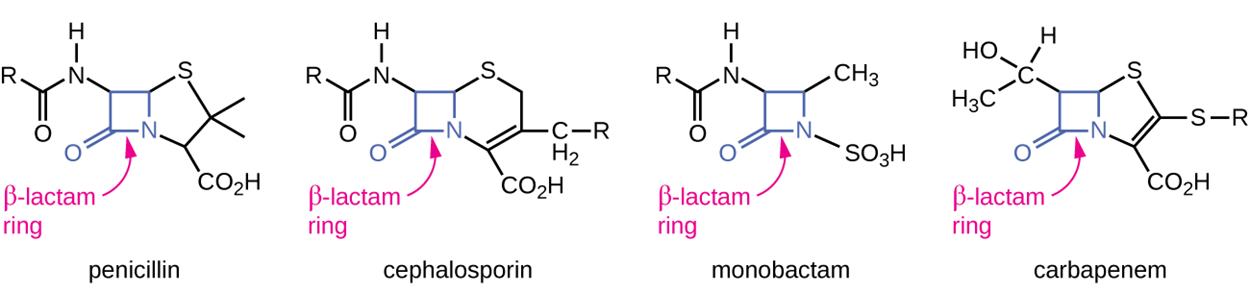
Cephalosporins also have β-lactam rings but have structural differences that help to protect them from inactivation by β-lactamases that damage the ring. Other medications that contain central β-lactam rings include carbapenems and monobactams.
Vancomycin, a class of glycopeptide, was discovered in the 1950s as a natural antibiotic from the actinomycete Amycolatopsis orientalis. This medication inhibits cell wall biosynthesis, although in a different manner from the β-lactams, and is also bactericidal against susceptible microbes (gram-positive pathogens). Vancomycin is a large molecule that binds to the end of the peptide chain of cell wall precursors, creating a structural blockage that prevents the cell wall subunits from being incorporated into the growing backbone of the peptidoglycan chain. Vancomycin cannot penetrate the outer membrane of gram-negative bacteria and is therefore not effective against them.
The drug bacitracin consists of a group of structurally similar peptide antibiotics originally isolated from Bacillus subtilis. This drug blocks the activity of a specific cell-membrane molecule that is responsible for the movement of peptidoglycan precursors from the cytoplasm to the exterior of the cell wall, preventing their incorporation into the cell wall. Bacitracin is effective against a range of bacteria but can damage the kidneys and is often combined with neomycin and polymyxin in topical ointments rather than taken orally.
The table below provides a more comprehensive overview of drugs that inhibit cell wall synthesis and the pathogens that they target.
| Drugs that Inhibit Bacterial Cell Wall Synthesis | ||||
|---|---|---|---|---|
| Mechanism of Action | Drug Class | Specific Drugs | Natural or Semisynthetic | Spectrum of Activity |
| Interact directly with PBPs and inhibit transpeptidase activity | Penicillins | Penicillin G, penicillin V | Natural | Narrow spectrum against gram-positive and a few gram-negative bacteria |
| Ampicillin, amoxicillin | Semisynthetic | Narrow spectrum against gram-positive bacteria but with increased gram-negative spectrum | ||
| Methicillin | Semisynthetic | Narrow spectrum against gram-positive bacteria only, including strains producing penicillinase | ||
| Cephalosporins | Cephalosporin C | Natural | Narrow spectrum similar to penicillin but with increased gram-negative spectrum | |
| First-generation cephalosporins | Semisynthetic | Narrow spectrum similar to cephalosporin C | ||
| Second-generation cephalosporins | Semisynthetic | Narrow spectrum but with increased gram-negative spectrum compared with first-generation cephalosporins | ||
| Third- and fourth-generation cephalosporins | Semisynthetic | Broad spectrum against gram-positive and gram-negative bacteria, including some β-lactamase producers | ||
| Fifth-generation cephalosporins | Semisynthetic | Broad spectrum against gram-positive and gram-negative bacteria, including Methicillin-resistant Staphylococcus aureus | ||
| Monobactams | Aztreonam | Semisynthetic | Narrow spectrum against gram-negative bacteria, including some β-lactamase producers | |
| Carbapenems | Imipenem, meropenem, doripenem | Semisynthetic | Broadest spectrum of the β-lactams against gram-positive and gram-negative bacteria, including many β-lactamase producers | |
| Large molecules that bind to the peptide chain of peptidoglycan subunits, blocking transglycosylation and transpeptidation | Glycopeptides | Vancomycin | Natural | Narrow spectrum against gram-positive bacteria only, including multidrug-resistant strains |
| Block transport of peptidoglycan subunits across cytoplasmic membrane | Bacitracin | Bacitracin | Natural | Broad-spectrum against gram-positive and gram-negative bacteria |
Bacterial cytoplasmic ribosomes differ from eukaryotic cytoplasmic ribosomes, making them another useful target for antimicrobial medications.
Some inhibitors of protein synthesis bind to the 30S subunit of bacterial ribosomes. Aminoglycosides inhibit the proofreading ability of the ribosomal complex, causing mismatches between codons and anticodons that ultimately result in the production of defective proteins. They are potent broad-spectrum antibacterial medications but can cause damage to the kidney, nervous system, and ear. Tetracyclines bind to the 30S subunit and block the association of tRNAs with the ribosome during translation. Natural and semisynthetic tetracyclines are available. They are broad spectrum but can cause negative effects such as phototoxicity, liver toxicity, and permanent discoloration of developing teeth when administered to children whose teeth are still developing.
Other inhibitors of protein synthesis bind to the 50S subunit. These include macrolides, which are broad-spectrum, bacteriostatic drugs that block protein elongation by inhibiting peptide bond formation between specific combinations of amino acids. Examples of these drugs are erythromycin and azithromycin. Lincosamides (including lincomycin and clindamycin) have a similar mode of action to macrolides and are especially effective against staphylococcal and streptococcal infections.
Chloramphenicol also binds to the 50S ribosomal subunit and inhibits peptide bond formation but can cause serious side effects in humans and is therefore now primarily used in veterinary medicine.
The oxazolidinones (such as linezolid) are a new class of broad-spectrum synthetic protein inhibitors that bind to the 50S ribosomal subunit. They appear to interfere with the formation of the initiation complex for translation and prevent translocation of the growing protein from the ribosomal A site to the ribosomal P site.
The table below summarizes important medications that target protein synthesis.
| Drugs That Inhibit Bacterial Protein Synthesis | |||||
|---|---|---|---|---|---|
| Molecular Target | Mechanism of Action | Drug Class | Specific Drugs | Bacteriostatic or Bactericidal | Spectrum of Activity |
| 30S subunit | Causes mismatches between codons and anticodons, leading to faulty proteins that insert into and disrupt cytoplasmic membrane | Aminoglycosides | Streptomycin, gentamicin, neomycin, kanamycin | Bactericidal | Broad spectrum |
| Blocks association of tRNAs with ribosome | Tetracyclines | Tetracycline, doxycycline, tigecycline | Bacteriostatic | Broad spectrum | |
| 50S subunit | Blocks peptide bond formation between amino acids | Macrolides | Erythromycin, azithromycin, telithromycin | Bacteriostatic | Broad spectrum |
| Lincosamides | Lincomycin, clindamycin | Bacteriostatic | Narrow spectrum | ||
| Not applicable | Chloramphenicol | Bacteriostatic | Broad spectrum | ||
| Interferes with the formation of the initiation complex between 50S and 30S subunits and other factors | Oxazolidinones | Linezolid | Bacteriostatic | Broad spectrum | |
A small group of antibacterial medications target the bacterial membrane as their mode of action. These include polymyxins, which interact with the lipopolysaccharide component of the outer membrane of gram-negative bacteria to disrupt both the outer and inner membranes to kill the cells. These medications can cause serious adverse effects by harming eukaryotic cell membranes as well, so they are used only in specific cases. At present, they are sometimes used as a last resort for antibiotic-resistant infections.
Another antibacterial, daptomycin, appears to have a similar mechanism of action but specifically targets gram-positive bacteria and appears to be better tolerated. It is typically administered intravenously.
The table below summarizes medications that inhibit bacterial membrane function.
| Drugs That Inhibit Bacterial Membrane Function | ||||
|---|---|---|---|---|
| Mechanism of Action | Drug Class | Specific Drugs | Spectrum of Activity | Clinical Use |
| Interacts with lipopolysaccharide in the outer membrane of gram-negative bacteria, killing the cell through the eventual disruption of the outer membrane and cytoplasmic membrane | Polymyxins | Polymyxin B | Narrow spectrum against gram-negative bacteria, including multidrug-resistant strains | Topical preparations to prevent infections in wounds |
| Polymyxin E (colistin) | Narrow spectrum against gram-negative bacteria, including multidrug-resistant strains | Oral dosing to decontaminate bowels to prevent infections in immunocompromised patients or patients undergoing invasive surgeries/procedures | ||
| Intravenous dosing to treat serious systemic infections caused by multidrug-resistant pathogens | ||||
| Inserts into the cytoplasmic membrane of gram-positive bacteria, disrupting the membrane and killing the cell | Lipopeptide | Daptomycin | Narrow spectrum against gram-positive bacteria, including multidrug-resistant strains | Complicated skin and skin-structure infections and bacteremia caused by gram-positive pathogens, including Methicillin-resistant S. aureus |
Another mechanism of action is inhibition of nucleic acid synthesis. Metronidazole, a member of the nitroimidazole family, is effective against bacteria and also acts against protozoa. It interferes with DNA replication in target cells. Rifampin is a semisynthetic member of the rifamycin family and blocks RNA polymerase activity in bacteria (remember that RNA polymerases differ in prokaryotes and eukaryotes). Rifampin is often used in medication cocktails to treat the mycobacteria that cause tuberculosis and can potentially have negative effects including interference with the therapeutic effect of other medications.
A member of the quinolone family (a group of synthetic antimicrobials) is nalidixic acid, which selectively inhibits the activity of DNA gyrase and therefore inhibits DNA replication. Chemical modifications of the quinolone backbone have led to the development of fluoroquinolones such as ciprofloxacin and levofloxacin, which are widely used and effective against a broad spectrum of gram-positive and gram-negative bacteria. Unfortunately, these medications still have some significant adverse effects. For example, among other possible effects, fluoroquinolones can cause damage to the nervous system and heart in addition to potentially affecting the metabolism of glucose.
The table below summarizes drugs that inhibit nucleic acid synthesis.
| Drugs That Inhibit Bacterial Nucleic Acid Synthesis | ||||
|---|---|---|---|---|
| Mechanisms of Action | Drug Class | Specific Drugs | Spectrum of Activity | Clinical Use |
| Inhibits bacterial RNA polymerase activity and blocks transcription, killing the cell | Rifamycin | Rifampin | Narrow spectrum with activity against gram-positive and limited numbers of gram-negative bacteria; also active against Mycobacterium tuberculosis | Combination therapy for treatment of tuberculosis |
| Inhibits the activity of DNA gyrase and blocks DNA replication, killing the cell | Fluoroquinolones | Ciprofloxacin, levofloxacin, ofloxacin, moxifloxacin | Broad spectrum against gram-positive and gram-negative bacteria | Wide variety of skin and systemic infections |
Some synthetic drugs control bacterial infections by functioning as antimetabolites (competitive inhibitors for bacterial metabolic enzymes). These include two major groups: inhibitors of folic acid synthesis and inhibitors of mycolic acid synthesis.
Sulfonamides (sulfa drugs) are the oldest synthetic antibacterial agents and block biosynthesis of folic acid, thereby inhibiting the production of purines and pyrimidines used to synthesize nucleic acids. This provides bacteriostatic inhibition of growth against a wide spectrum of gram-positive and gram-negative bacteria. Although these medications have good selective toxicity because humans synthesize folic acid from food, allergic reactions are common.
Trimethoprim is an antimicrobial compound that serves as an antimetabolite within the same folic acid synthesis pathway as sulfonamides but inhibits a later step in the metabolic pathway. It is often used in combination with the sulfa drug sulfamethoxazole to treat urinary tract infections, ear infections, and bronchitis. These medications together produce bactericidal instead of bacteriostatic effects.
Izoniazid is an antimetabolite with a specific toxicity for mycobacteria that is often used in combination with rifampin or streptomycin to treat tuberculosis. It is administered as a prodrug, meaning it must be activated by an intracellular bacterial peroxidase enzyme to form izoniazid-nicotinamide adenine dinucleotide (NAD) and izoniazid-nicotinamide adenine dinucleotide phosphate (NADP). This inhibits the synthesis of mycolic acid, which is essential for mycobacterial cell walls. However, it can cause potentially significant adverse effects such as liver toxicity, nervous system toxicity (neurotoxicity), and hematologic toxicity (anemia).
The table below summarizes antimetabolic medications.
| Antimetabolite Drugs | ||||
|---|---|---|---|---|
| Metabolic Pathway Target | Mechanism of Action | Drug Class | Specific Drugs | Spectrum of Activity |
| Folic acid synthesis | Inhibits the enzyme involved in production of dihydrofolic acid | Sulfonamides | Sulfamethoxazole | Broad spectrum against gram-positive and gram-negative bacteria |
| Sulfones | Dapsone | |||
| Inhibits the enzyme involved in the production of tetrahydrofolic acid | Not applicable | Trimethoprim | Broad spectrum against gram-positive and gram-negative bacteria | |
| Mycolic acid synthesis | Interferes with the synthesis of mycolic acid | Not applicable | Izoniazid | Narrow spectrum against Mycobacterium spp., including M. tuberculosis |
At present, there is a single antimicrobial agent (bedaquiline) that appears to interfere with ATP synthase function. Bedaquiline is a member of the synthetic antibacterial class of compounds called diarylquinolines. This medication is currently only used for serious, otherwise untreatable cases of tuberculosis as it is associated with potentially severe adverse effects. For example, these medications can cause heart arrhythmia (irregular heartbeats) that can potentially be lethal. Additionally, these medications sometimes harm the liver.
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “MICROBIOLOGY.” ACCESS FOR FREE AT openstax.org/details/books/microbiology. LICENSE: CC ATTRIBUTION 4.0 INTERNATIONAL.
REFERENCES
Britannica, T. Editors of Encyclopaedia (2019, November 19). therapeutic index. Encyclopedia Britannica. Retrieved November 15, 2022, from www.britannica.com/science/therapeutic-index