Table of Contents |
Recall from previous lessons that lipids are nonpolar molecules that are hydrophobic (“water-fearing”), or insoluble in water. Lipids perform many different functions in a cell. Cells store energy for long-term use in the form of fats. Lipids also provide insulation from the environment, are the building blocks of many hormones, and are an important constituent of all cellular membranes. Lipids include fats, waxes, phospholipids, and steroids.
Fats (or triglycerides) within the body are ingested as food or synthesized by adipocytes or hepatocytes from carbohydrate precursors. Lipid metabolism entails the oxidation of fatty acids to either generate energy or synthesize new lipids from smaller constituent molecules. Lipid metabolism is associated with carbohydrate metabolism, as products of glucose (such as acetyl-CoA) can be converted into lipids.
Lipid metabolism begins in the intestine where ingested triglycerides are broken down into smaller chain fatty acids and subsequently into monoglyceride molecules (see panel b of the image below) by pancreatic lipases, which are enzymes that break down fats after they are emulsified by bile salts. When food reaches the small intestine in the form of chyme, a digestive hormone called cholecystokinin (CCK) is released by intestinal cells in the intestinal mucosa. CCK stimulates the release of pancreatic lipase from the pancreas and stimulates the contraction of the gallbladder to release stored bile salts into the intestine. CCK also travels to the brain, where it can act as a hunger suppressant.

Together, the pancreatic lipases and bile salts break down triglycerides into free fatty acids. These fatty acids can be transported across the intestinal membrane. However, once they cross the membrane, they are recombined to again form triglyceride molecules. Within the intestinal cells, these triglycerides are packaged along with cholesterol molecules in phospholipid vesicles called chylomicrons (see the image below).
The chylomicrons enable fats and cholesterol to move within the aqueous environment of your lymphatic and circulatory systems. Chylomicrons leave the enterocytes by exocytosis and enter the lymphatic system via lacteals in the villi of the intestine. From the lymphatic system, the chylomicrons are transported to the circulatory system. Once in circulation, they can either go to the liver or be stored in fat cells (adipocytes) that comprise adipose (fat) tissue found throughout the body.

To obtain energy from fat, triglycerides must first be broken down by hydrolysis into their two principal components, fatty acids and glycerol. This process, called lipolysis, takes place in the cytoplasm of a cell. The resulting fatty acids are oxidized by β-oxidation into acetyl-CoA, which you previously learned is used by the citric acid cycle. The glycerol that is released from triglycerides after lipolysis directly enters the glycolysis pathway as DHAP.
Because one triglyceride molecule yields three fatty acid molecules with as much as 16 or more carbons in each one, fat molecules yield more energy than carbohydrates and are an important source of energy for the human body. Triglycerides yield more than twice the energy per unit mass compared with carbohydrates and proteins. Therefore, when glucose levels are low, triglycerides can be converted into acetyl-CoA molecules and used to generate ATP through aerobic respiration.
The breakdown of fatty acids, called fatty acid oxidation or beta (β)-oxidation, begins in the cytoplasm, where fatty acids are converted into fatty acyl-CoA molecules. This fatty acyl-CoA combines with carnitine to create a fatty acyl carnitine molecule, which helps to transport the fatty acid across the mitochondrial membrane. Once inside the mitochondrial matrix, the fatty acyl carnitine molecule is converted back into fatty acyl-CoA and then into acetyl-CoA. The newly formed acetyl-CoA enters the citric acid cycle and is used to produce ATP in the same way as acetyl-CoA derived from pyruvate.
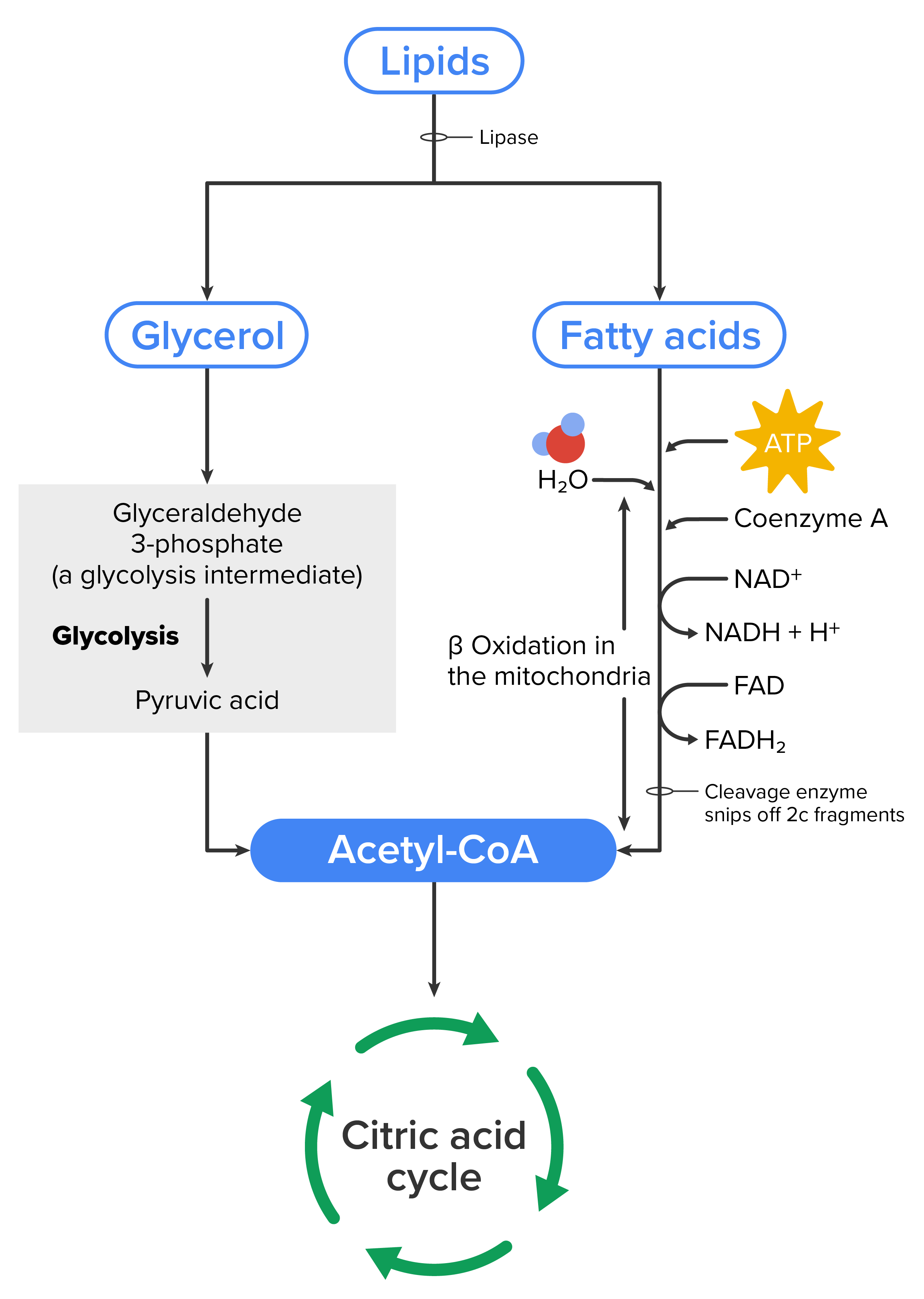
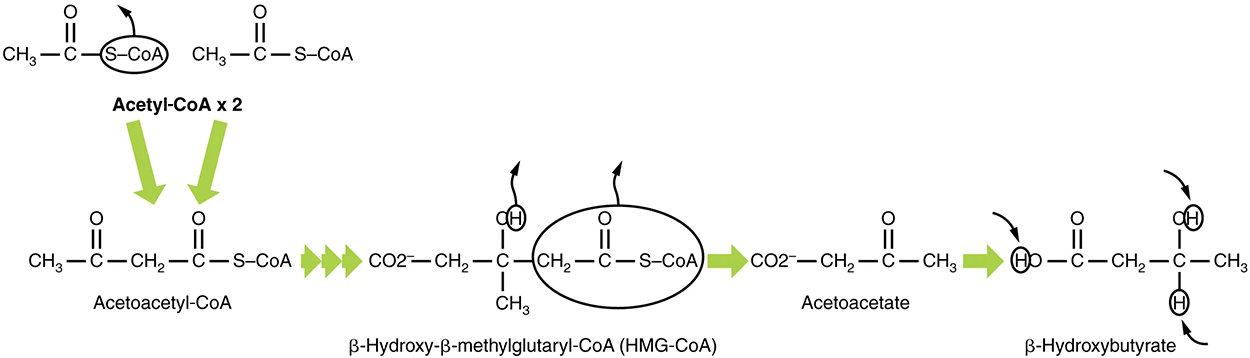
If excessive acetyl-CoA is created from the oxidation of fatty acids and the citric acid cycle is overloaded and cannot handle it, the acetyl-CoA is diverted to create ketone bodies through ketogenesis.
In this ketone synthesis reaction, excess acetyl-CoA is converted into hydroxymethylglutaryl-CoA (HMG-CoA). HMG-CoA is a precursor of cholesterol and is an intermediate that is subsequently converted into β-hydroxybutyrate, the primary ketone body in the blood.
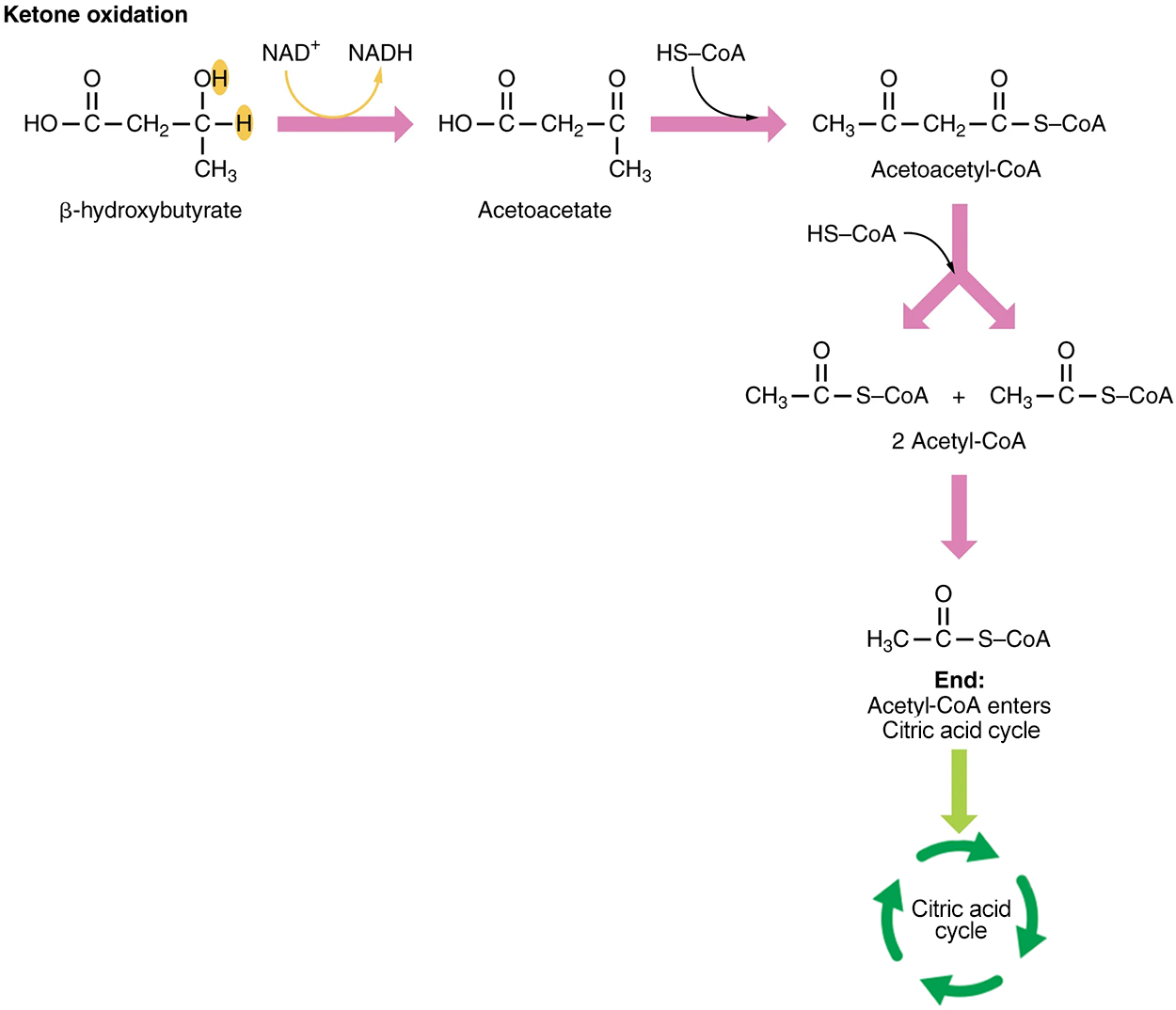
Organs that have classically been thought to be dependent solely on glucose, such as the brain, can actually use ketones as an alternative energy source. This keeps the brain functioning when glucose is limited. When ketones are produced faster than they can be used, they can be broken down into CO₂ and acetone. The acetone is removed by exhalation.
Ketones oxidize to produce energy for the brain. Beta (β)-hydroxybutyrate is oxidized to acetoacetate, and NADH is released. An HS-CoA molecule (this is a way that coenzyme A is written) is added to acetoacetate, forming acetoacetyl-CoA. The carbon within the acetoacetyl-CoA that is not bonded to the CoA then detaches, splitting the molecule in two. This carbon then attaches to another free HS-CoA, resulting in two acetyl-CoA molecules. These two acetyl-CoA molecules are then processed through the citric acid cycle to generate energy.
When glucose levels are plentiful, the excess acetyl-CoA generated by glycolysis can be converted into fatty acids, triglycerides, cholesterol, steroids, and bile salts. This process, called lipogenesis, creates lipids (fat) from the acetyl-CoA and takes place in the cytoplasm of adipocytes (fat cells) and hepatocytes (liver cells). When you eat more glucose or carbohydrates than your body needs, your system uses acetyl-CoA to turn the excess into fat.
Although there are several metabolic sources of acetyl-CoA, it is most commonly derived from glycolysis. Acetyl-CoA availability is important because it initiates lipogenesis. Lipogenesis begins with acetyl-CoA and advances by the subsequent addition of two carbon atoms from another acetyl-CoA; this process is repeated until fatty acids are the appropriate length. Because this is a bond-creating anabolic process, ATP is consumed. However, the creation of triglycerides and other lipids is an efficient way of storing the energy available in carbohydrates. Triglycerides and other lipids, which are high-energy molecules, are stored in adipose tissue until they are needed.
In lipid metabolism, there are different pathways that lipids may follow.
EXAMPLE
Through catabolic reactions, glycerol is formed by lipolysis of triglycerides and can then proceed to undergo glycolysis. However, fatty acids undergo β-oxidation, which creates acetyl-CoA that then undergoes the citric acid cycle to produce ATP, or ketogenesis to produce ketone bodies. The reverse of these processes also occurs by anabolic reactions.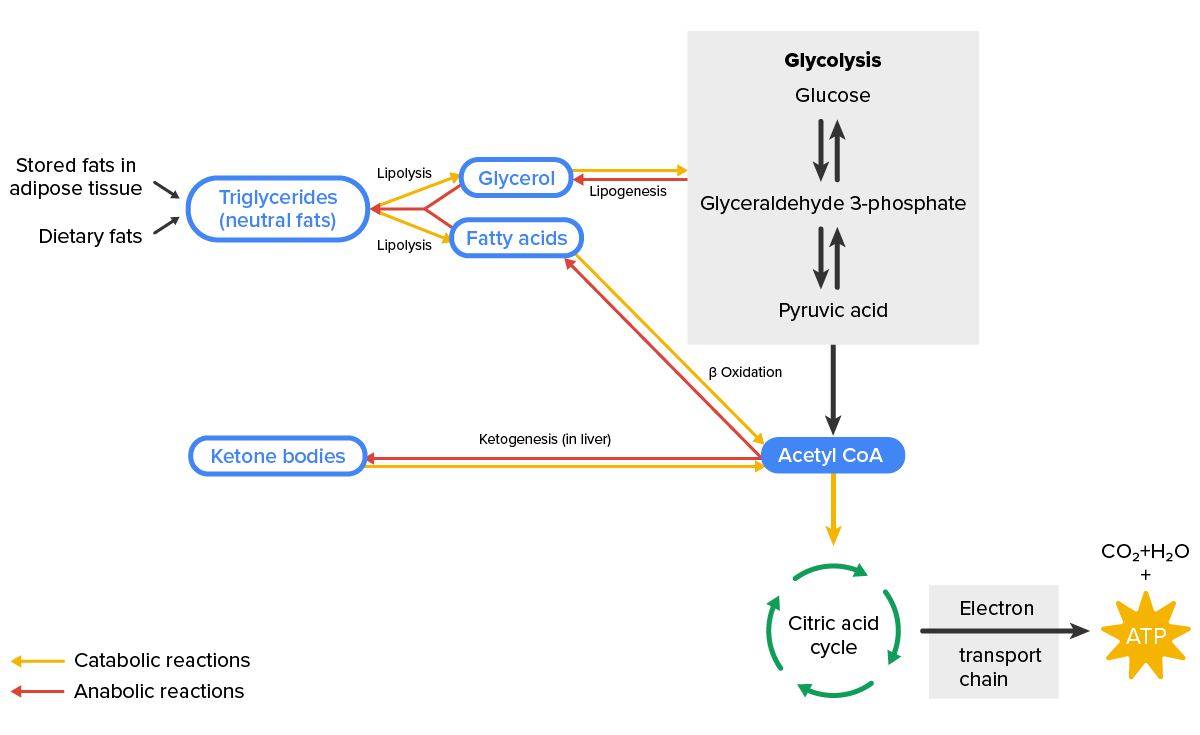
SOURCE: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “ANATOMY AND PHYSIOLOGY 2E”. ACCESS FOR FREE AT OPENSTAX.ORG/BOOKS/ANATOMY-AND-PHYSIOLOGY-2E/PAGES/1-INTRODUCTION. LICENSE: CREATIVE COMMONS ATTRIBUTION 4.0 INTERNATIONAL.