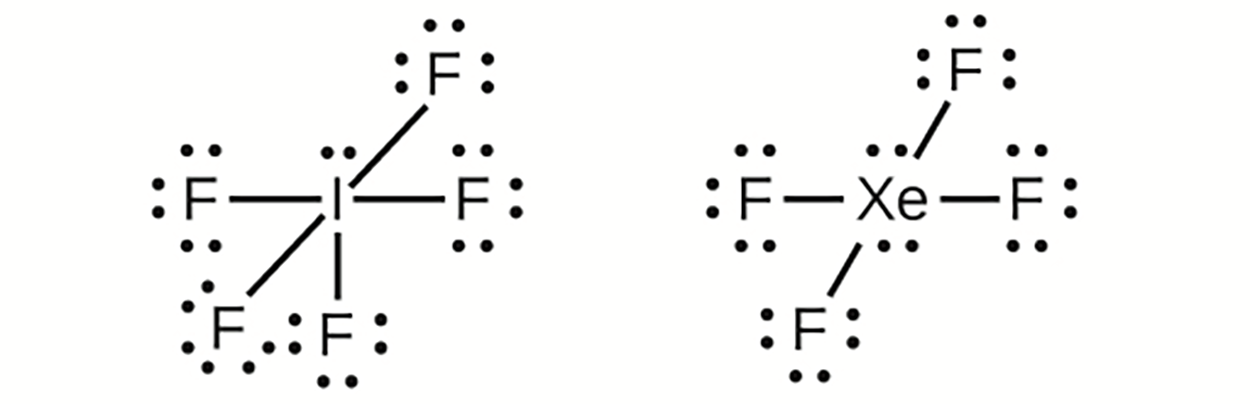In this lesson, you will learn about using Lewis symbols to draw Lewis structures. Specifically, this lesson covers:
1. Lewis Symbols and Lewis Structures
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons as the image below shows:

![The Lewis symbols for the elements of the third period of the periodic table. able showing atoms, Electronic Configuration, and the Lewis Symbol. The sodium atom has an electronic configuration of [NE]3s1 and the Lewis Symbol is Na plus one dot to represent the one valence electron. Magnesium [Ne]3s2 and the Lewis Symbol is Mg plus two dots to represent the two valence electrons. The two dots are not paired up but shown singly on either side of the Mg. Aluminum [Ne]3s23p1 and the Lewis Symbol is Al plus three dots to represent the three valence electrons. The three dots are not paired up but shown singly around the Al. Silicon [Ne]3s23p3 and the Lewis Symbol is Si plus four dots to represent the four valence electrons. The four dots are not paired up but shown singly around the Si. Phosphorus [Ne]3s23p3 and the Lewis Symbol is P plus five dots to represent the five valence electrons. The five dots are arranged with one pair of dots and three single dots around the P. Sulfur [Ne]3s23p4 and the Lewis Symbol is S plus six dots to represent the six valence electrons. The six dots are arranged with two pairs of dots and two single dots around the S. Chlorine [Ne]3s23p5 and the Lewis Symbol is Cl plus seven dots to represent the seven valence electrons. The seven dots are arranged with three pairs of dots and a single dot around the Cl. Argon [Ne]3s23p6 and the Lewis Symbol is Ar plus eight dots to represent the eight valence electrons. The eight dots are arranged with four pairs of dots around the Ar.](https://cdn2.sophia.org/markup_pictures/12668/file/955532d1a379f8f2e36a45590b3b57c8.png)
-
Lewis symbols illustrate the number of valence electrons for each element in the third period of the periodic table.


Lewis symbols can be used to show the transfer of electrons during the formation of ionic compounds.
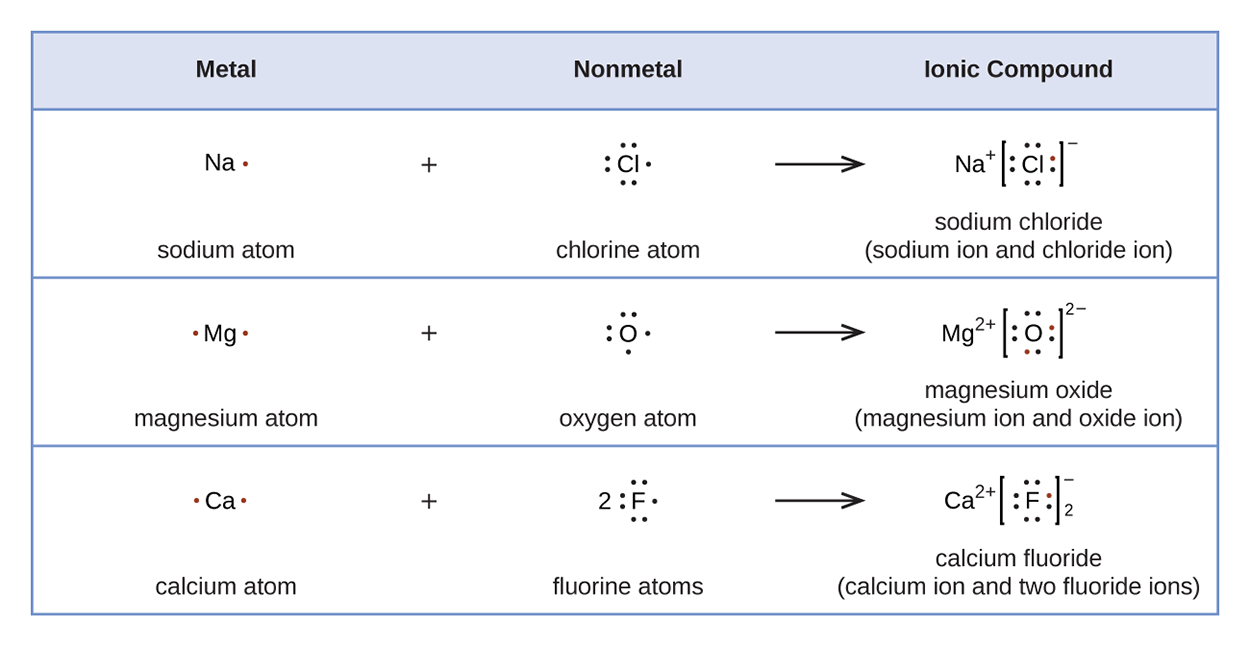
Cations are formed when atoms lose electrons, represented by fewer Lewis dots, whereas anions are formed by atoms gaining electrons. The total number of electrons does not change.
We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures, drawings that describe the bonding in molecules and polyatomic ions. For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons.
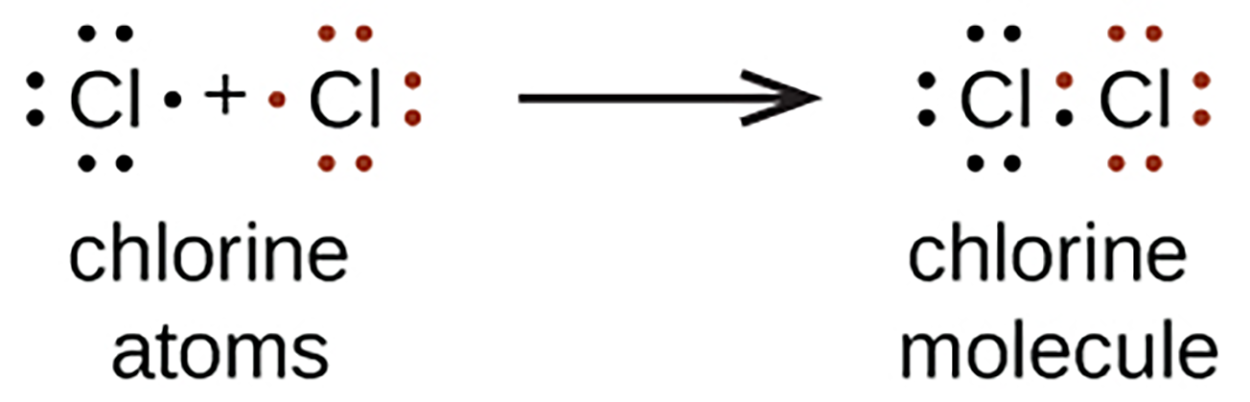
The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). A dash (or line) is sometimes used to indicate a shared pair of electrons.

A single shared pair of electrons is called a single bond. Each Cl atom interacts with eight valence electrons: the six in the lone pairs and the two in the single bond.
-
- Lewis Symbol
- A elemental symbol surrounded by one dot for each of its valence electrons.
- Lewis Structure
- A drawing that describes the bonding in molecules and polyatomic ions.
- lone Pair
- A pair of electrons that are not used in bonding.
- Single Bond
- When a pair of atoms share one pair of electrons.
2. The Octet Rule
The tendency of main group atoms to form enough bonds to obtain eight valence electrons is known as the octet rule. The number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight valence electrons); this is especially true of the nonmetals of the second period of the periodic table (C, N, O, and F).
-
EXAMPLE
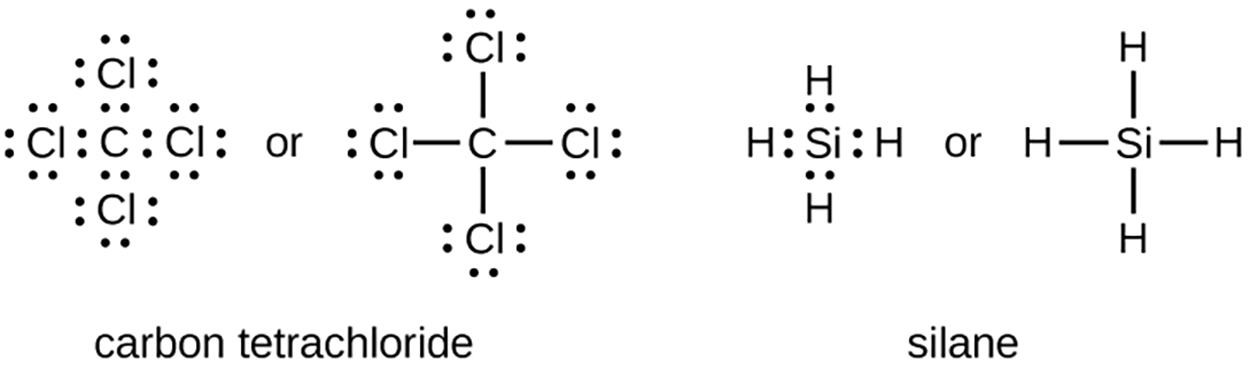
Each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. These four electrons can be gained by forming four covalent bonds, as seen below for carbon in CCl

(carbon tetrachloride) and silicon in SiH

(silane). Because hydrogen only needs two electrons to fill its valence shell, it is an exception to the octet rule.
-
EXAMPLE
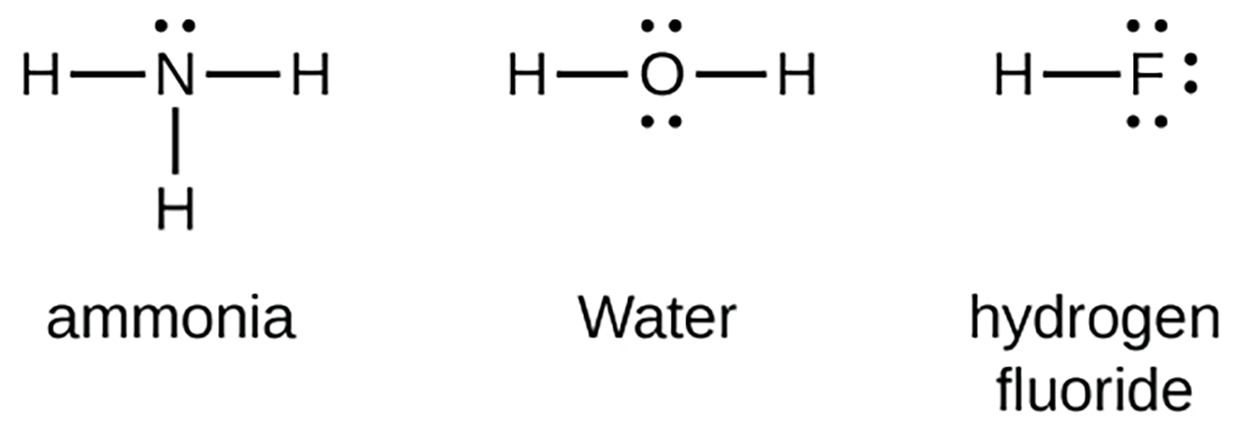
Group 15 elements such as nitrogen have five valence electrons in the atomic Lewis symbol: one lone pair and three unpaired electrons. To obtain an octet, these atoms form three covalent bonds, as in NH

(ammonia). Oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds and the halogens and other atoms in group 17 obtain an octet by forming one covalent bond.
-
- Octet Rule
- The tendency of main group atoms to form enough bonds to obtain eight valence electrons.
3. Multiple Bonds
When a pair of atoms share one pair of electrons, we call this a single bond. However, a pair of atoms may need to share more than one pair of electrons in order to achieve the requisite octet. A double bond forms when two pairs of electrons are shared between a pair of atoms, as between the carbon and oxygen atoms in CH O (formaldehyde) and between the two carbon atoms in C2H
O (formaldehyde) and between the two carbon atoms in C2H (ethylene).
(ethylene).
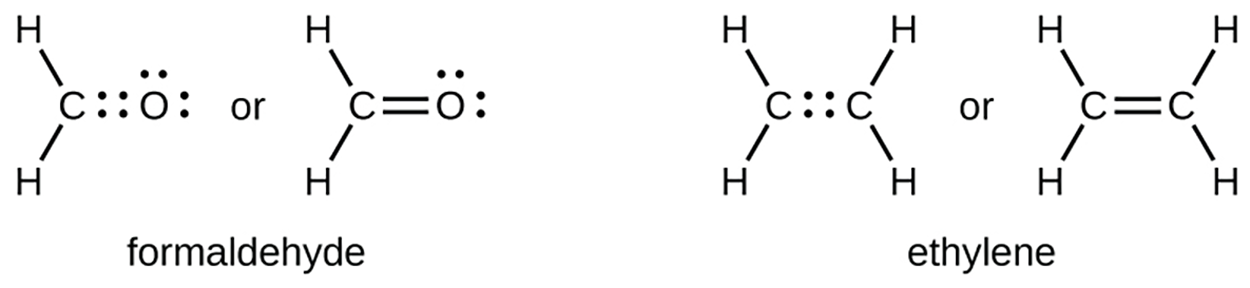
A triple bond forms when three electron pairs are shared by a pair of atoms, as in carbon monoxide (CO) and the cyanide ion (CN ).
).

-
- Double Bond
- When two pairs of electrons are shared between a pair of atoms.
- Triple Bond
- When three electron pairs are shared by a pair of atoms.
4. Writing Lewis Structures
For very simple molecules and molecular ions, we can write the Lewis structures by merely pairing up the unpaired electrons on the constituent atoms.
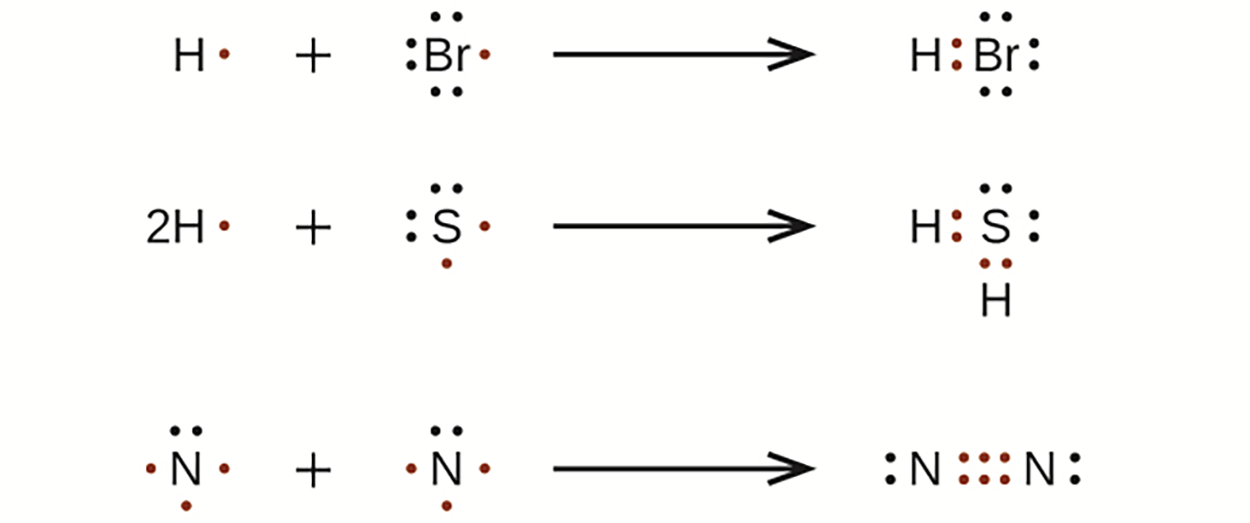
-
For more complicated molecules and molecular ions, it is helpful to follow the step-by-step procedure outlined here:
- Determine the total number of valence (outer shell) electrons. For cations, subtract one electron for each positive charge. For anions, add one electron for each negative charge.
- Draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom. (Generally, the least electronegative element should be placed in the center.) Connect each atom to the central atom with a single bond (one electron pair).
- Distribute the remaining electrons as lone pairs on the terminal atoms (except hydrogen), completing an octet around each atom.
- Place all remaining electrons on the central atom.
- Rearrange the electrons of the outer atoms to make multiple bonds with the central atom in order to obtain octets wherever possible.
-
EXAMPLE
Draw the Lewis structures of the molecule, SiH

.
Solution:
1. Determine the total number of valence (outer shell) electrons in the molecule or ion.
For a molecule, we add the number of valence electrons on each atom in the molecule:
Silicon is in group 14, so it has 4 valence electrons. Hydrogen is in group 1, so each hydrogen has 1 valence electron. There are 4 hydrogen atoms, so there is a total of 4 valence electrons from hydrogen and 4 from silicon for a total of 8 valence electrons.
2. Draw a skeleton structure of the molecule, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond.

3. Distribute the remaining electrons as lone pairs on the terminal atoms (except hydrogen) to complete their valence shells with an octet of electrons.
There are no remaining electrons in SiH

, so it is unchanged.
4. Place all remaining electrons on the central atom.
For SiH

, there are no remaining electrons; we already placed all of the electrons determined in Step 1.
5. Rearrange the electrons of the outer atoms to make multiple bonds with the central atom in order to obtain octets wherever possible.
SiH

: Si already has an octet, so nothing needs to be done.
-
EXAMPLE
Draw the Lewis structures of the ion, CHO
-.
Solution:
1. Determine the total number of valence (outer shell) electrons in the molecule or ion.
For a negative ion, we add the number of valence electrons on each atom in the molecule to the number of negative changes on the ion (one electron is gained for each single negative charge):
Carbon is in group 14, so it has 4 valence electrons. Hydrogen is in group 1, so each hydrogen has 1 valence electron. Oxygen is in group 16, so it has 6 valence electrons. There are 2 oxygen atoms, so there are a total of 12 valence electrons from oxygen. There is a 1- charge, so there is one additional electron. This is a total of 18 valence electrons.
2. Draw a skeleton structure of the ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. (Note that we denote ions with brackets around the structure, indicating the charge outside the brackets:)
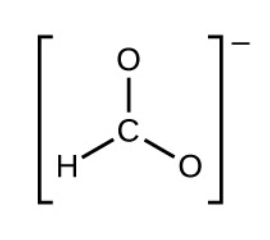
3. Distribute the remaining electrons as lone pairs on the terminal atoms (except hydrogen) to complete their valence shells with an octet of electrons.
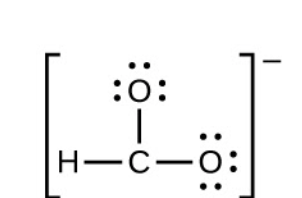
4. Place all remaining electrons on the central atom.
For CHO
-, there are no remaining electrons; we already placed all of the electrons determined in Step 3.
5. Rearrange the electrons of the outer atoms to make multiple bonds with the central atom in order to obtain octets wherever possible.
For CHO
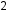
-, We have distributed the valence electrons as lone pairs on the oxygen atoms, but the carbon atom lacks an octet:
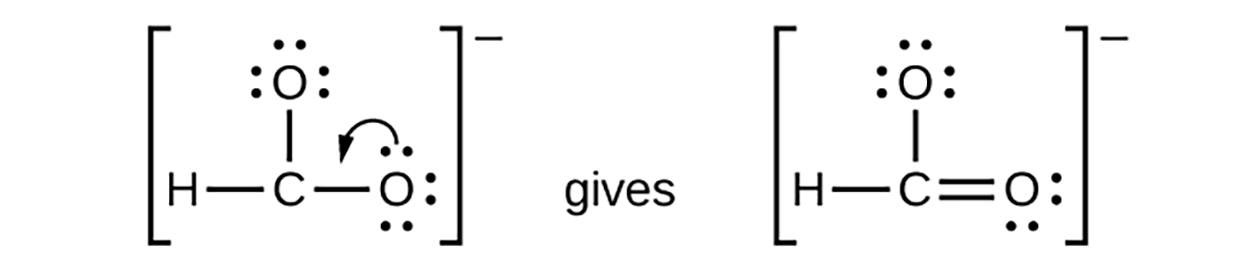
5. Exceptions to the Octet Rule
Many covalent molecules have central atoms that do not have eight electrons in their Lewis structures. These molecules fall into three categories:
- Odd-electron molecules have an odd number of valence electrons and therefore have an unpaired electron.
- Electron-deficient molecules have a central atom that has fewer electrons than needed for a noble gas configuration.
- Hypervalent molecules have a central atom that has more electrons than needed for a noble gas configuration.
We call molecules that contain an odd number of electrons
free radicals. Nitric oxide, NO, is an example of an odd-electron molecule; it is produced in internal combustion engines when oxygen and nitrogen react at high temperatures.
-
To draw the Lewis structure for an odd-electron molecule like NO, we follow the same five steps we would for other molecules, but with a few minor changes:
1.
Determine the total number of valence (outer shell) electrons. The sum of the valence electrons is 5 (from N) + 6 (from O) = 11. The odd number immediately tells us that we have a free radical, so we know that not every atom can have eight electrons in its valence shell.
2.
Draw a skeleton structure of the molecule. We can easily draw a skeleton with an N–O single bond:
N–O
3.
Distribute the remaining electrons as lone pairs on the terminal atoms. In this case, there is no central atom, so we distribute the electrons around both atoms. We give eight electrons to the more electronegative atom in these situations; thus oxygen has the filled valence shell:

4.
Place all remaining electrons on the central atom. Since there are no remaining electrons, this step does not apply.
5.
Rearrange the electrons to make multiple bonds with the central atom in order to obtain octets wherever possible. We know that an odd-electron molecule cannot have an octet for every atom, but we want to get each atom as close to an octet as possible. In this case, nitrogen has only five electrons around it. To move closer to an octet for nitrogen, we take one of the lone pairs from oxygen and use it to form a NO double bond. (We cannot take another lone pair of electrons on oxygen and form a triple bond because nitrogen would then have nine electrons:)

We will also encounter a few molecules that contain central atoms that do not have a filled valence shell. Generally, these are molecules with central atoms from groups 2 and 13, outer atoms that are hydrogen, or other atoms that do not form multiple bonds.
-
EXAMPLE
in the Lewis structures of beryllium dihydride, BeH

, and boron trifluoride, BF

, the beryllium and boron atoms each have only four and six electrons, respectively. It is possible to draw a structure with a double bond between a boron atom and a fluorine atom in BF

, satisfying the octet rule, but experimental evidence indicates the bond lengths are closer to that expected for B–F single bonds.
This suggests the best Lewis structure has three B–F single bonds and an electron-deficient boron. The reactivity of the compound is also consistent with an electron-deficient boron. However, the B–F bonds are slightly shorter than what is actually expected for B–F single bonds, indicating that some double bond character is found in the actual molecule.
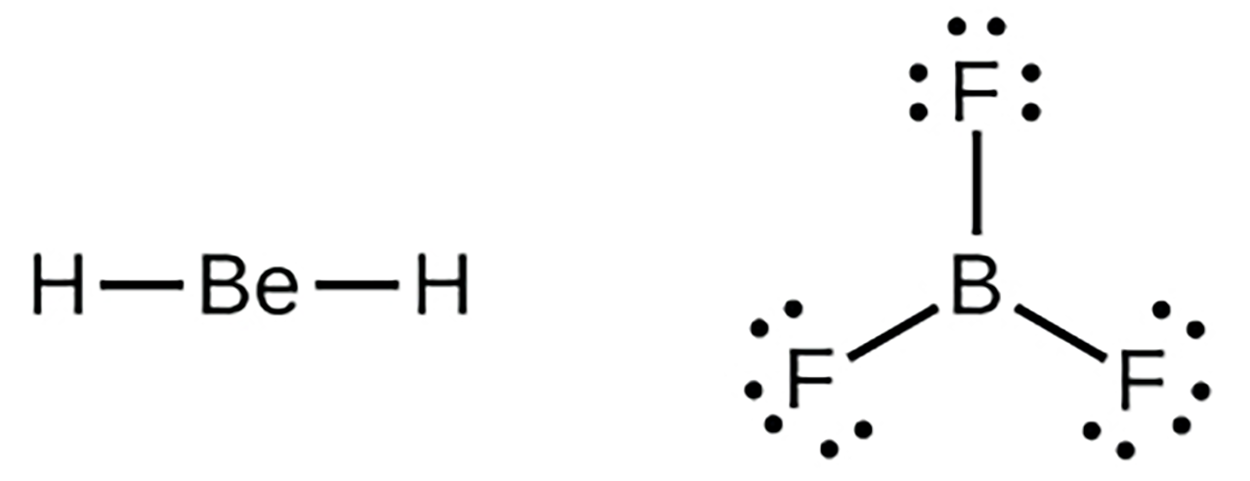
An atom like the boron atom in BF , which does not have eight electrons, is very reactive. It readily combines with a molecule containing an atom with a lone pair of electrons. For example, NH
, which does not have eight electrons, is very reactive. It readily combines with a molecule containing an atom with a lone pair of electrons. For example, NH reacts with BF
reacts with BF because the lone pair on nitrogen can be shared with the boron atom:
because the lone pair on nitrogen can be shared with the boron atom:
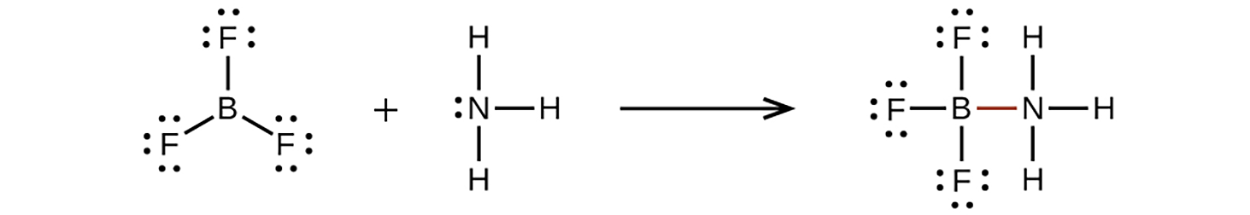
Elements in the second period of the periodic table can accommodate only eight electrons in their valence shell orbitals because they have only four valence orbitals (one 2s and three 2p orbitals). Elements in the third and higher periods have more than four valence orbitals and can share more than four pairs of electrons with other atoms, because they have empty d orbitals in the same shell. Molecules formed from these elements are sometimes called hypervalent molecules.
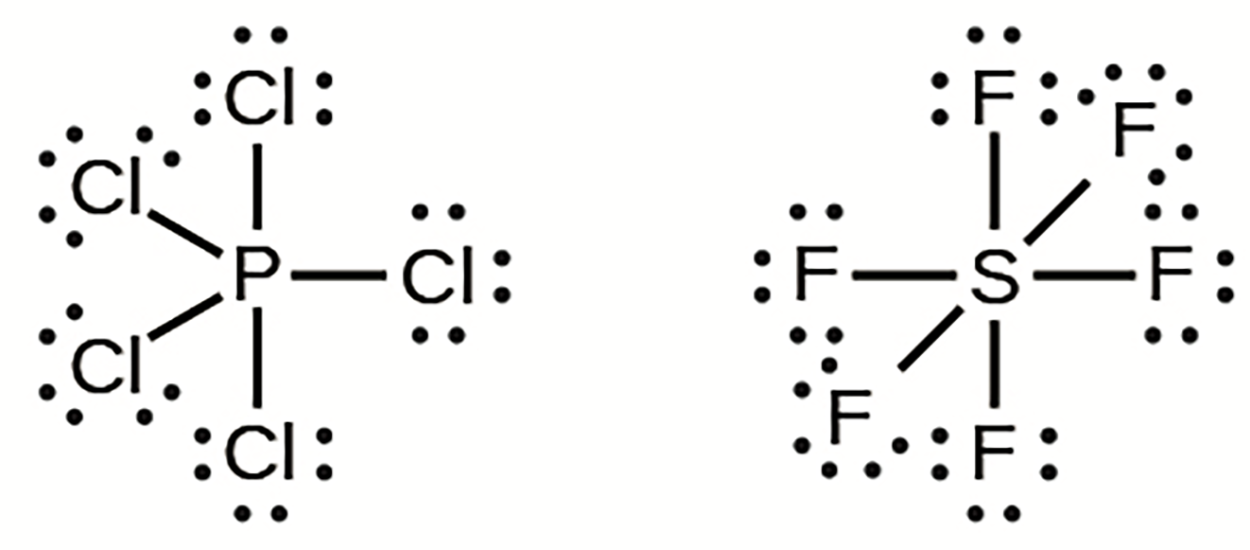
In PCl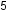 , the central atom phosphorus shares five pairs of electrons. In SF
, the central atom phosphorus shares five pairs of electrons. In SF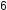 , sulfur shares six pairs of electrons.
, sulfur shares six pairs of electrons.
In some hypervalent molecules, such as IF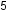 and XeF
and XeF , some of the electrons in the outer shell of the central atom are lone pairs:
, some of the electrons in the outer shell of the central atom are lone pairs:
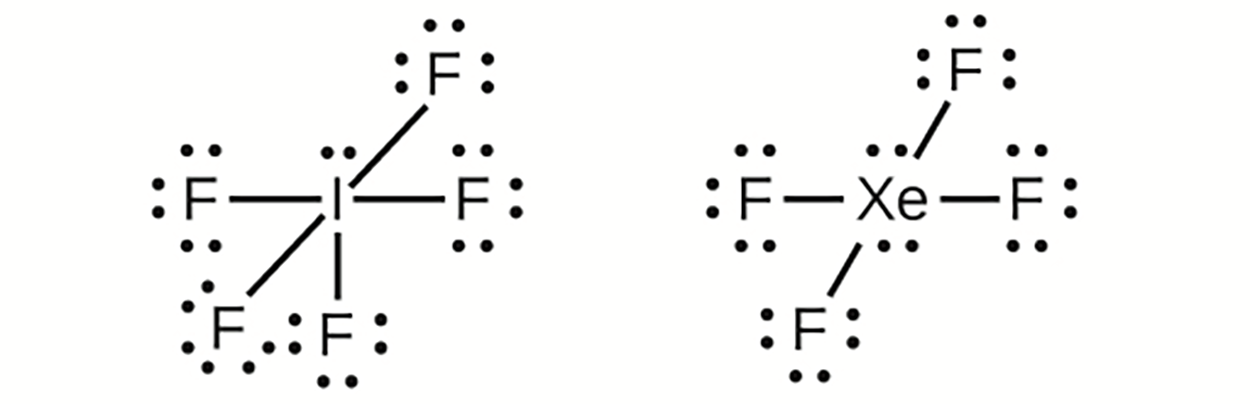
When we write the Lewis structures for these molecules, we find that we have electrons left over after filling the valence shells of the outer atoms with eight electrons. These additional electrons must be assigned to the central atom.
-
- Free Radical
- A molecule that contains an odd number of electrons.
- Hypervalent Molecule
- A molecule that is formed from elements in the third and higher periods and has more than four valence orbitals and can share more than four pairs of electrons with other atoms because they have empty d orbitals in the same shell.
In this lesson, you learned how to draw Lewis symbols for elements and how they can be used to write Lewis structures, which are a symbolic model of an element that shows bonds and lone pairs. You then learned to use the octet rule, to draw Lewis structures for compounds and ions that contain both single bonds and multiple bonds. You also learned how to identify elements that can make molecules that are an exception to the octet rule.
Best of luck in your learning!

![The Lewis symbols for the elements of the third period of the periodic table. able showing atoms, Electronic Configuration, and the Lewis Symbol. The sodium atom has an electronic configuration of [NE]3s1 and the Lewis Symbol is Na plus one dot to represent the one valence electron. Magnesium [Ne]3s2 and the Lewis Symbol is Mg plus two dots to represent the two valence electrons. The two dots are not paired up but shown singly on either side of the Mg. Aluminum [Ne]3s23p1 and the Lewis Symbol is Al plus three dots to represent the three valence electrons. The three dots are not paired up but shown singly around the Al. Silicon [Ne]3s23p3 and the Lewis Symbol is Si plus four dots to represent the four valence electrons. The four dots are not paired up but shown singly around the Si. Phosphorus [Ne]3s23p3 and the Lewis Symbol is P plus five dots to represent the five valence electrons. The five dots are arranged with one pair of dots and three single dots around the P. Sulfur [Ne]3s23p4 and the Lewis Symbol is S plus six dots to represent the six valence electrons. The six dots are arranged with two pairs of dots and two single dots around the S. Chlorine [Ne]3s23p5 and the Lewis Symbol is Cl plus seven dots to represent the seven valence electrons. The seven dots are arranged with three pairs of dots and a single dot around the Cl. Argon [Ne]3s23p6 and the Lewis Symbol is Ar plus eight dots to represent the eight valence electrons. The eight dots are arranged with four pairs of dots around the Ar.](https://cdn2.sophia.org/markup_pictures/12668/file/955532d1a379f8f2e36a45590b3b57c8.png)





 (carbon tetrachloride) and silicon in SiH
(carbon tetrachloride) and silicon in SiH (silane). Because hydrogen only needs two electrons to fill its valence shell, it is an exception to the octet rule.
(silane). Because hydrogen only needs two electrons to fill its valence shell, it is an exception to the octet rule.

 (ammonia). Oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds and the halogens and other atoms in group 17 obtain an octet by forming one covalent bond.
(ammonia). Oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds and the halogens and other atoms in group 17 obtain an octet by forming one covalent bond.

 O (formaldehyde) and between the two carbon atoms in C2H
O (formaldehyde) and between the two carbon atoms in C2H (ethylene).
(ethylene).

 ).
).


 .
.

 , so it is unchanged.
, so it is unchanged.
 , there are no remaining electrons; we already placed all of the electrons determined in Step 1.
, there are no remaining electrons; we already placed all of the electrons determined in Step 1.
 : Si already has an octet, so nothing needs to be done.
: Si already has an octet, so nothing needs to be done. -.
-.


 -, there are no remaining electrons; we already placed all of the electrons determined in Step 3.
-, there are no remaining electrons; we already placed all of the electrons determined in Step 3.
 -, We have distributed the valence electrons as lone pairs on the oxygen atoms, but the carbon atom lacks an octet:
-, We have distributed the valence electrons as lone pairs on the oxygen atoms, but the carbon atom lacks an octet:
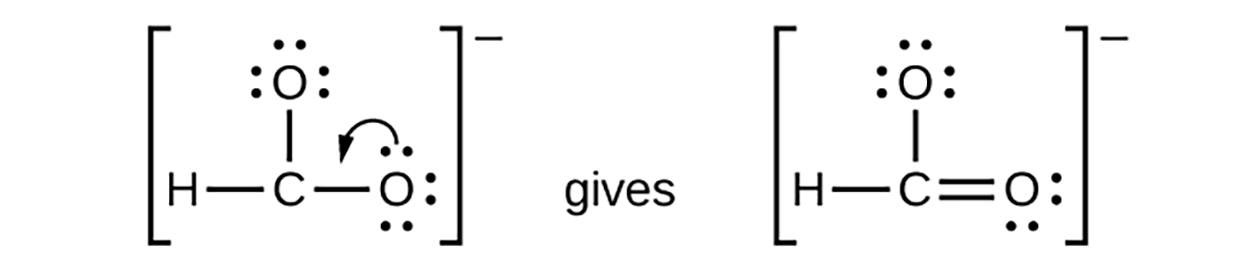


 , and boron trifluoride, BF
, and boron trifluoride, BF , the beryllium and boron atoms each have only four and six electrons, respectively. It is possible to draw a structure with a double bond between a boron atom and a fluorine atom in BF
, the beryllium and boron atoms each have only four and six electrons, respectively. It is possible to draw a structure with a double bond between a boron atom and a fluorine atom in BF , satisfying the octet rule, but experimental evidence indicates the bond lengths are closer to that expected for B–F single bonds.
, satisfying the octet rule, but experimental evidence indicates the bond lengths are closer to that expected for B–F single bonds.
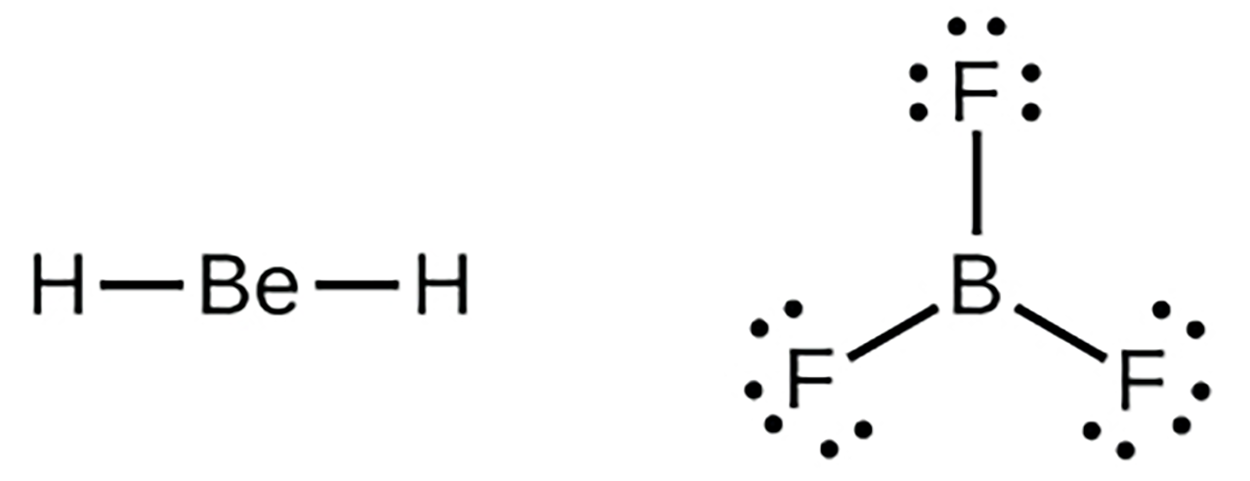
 , which does not have eight electrons, is very reactive. It readily combines with a molecule containing an atom with a lone pair of electrons. For example, NH
, which does not have eight electrons, is very reactive. It readily combines with a molecule containing an atom with a lone pair of electrons. For example, NH reacts with BF
reacts with BF because the lone pair on nitrogen can be shared with the boron atom:
because the lone pair on nitrogen can be shared with the boron atom:
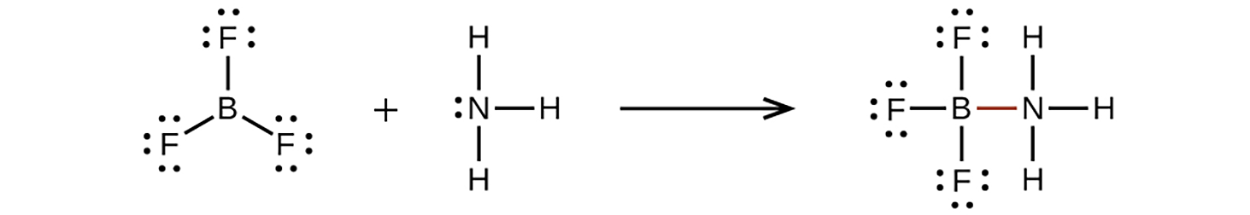
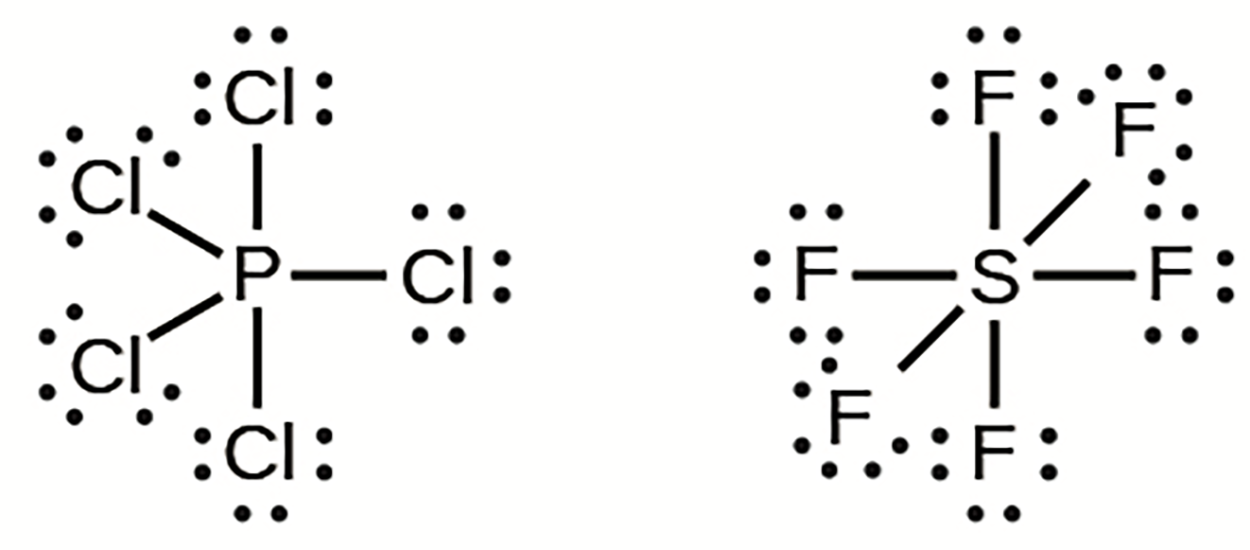
 , the central atom phosphorus shares five pairs of electrons. In SF
, the central atom phosphorus shares five pairs of electrons. In SF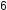 , sulfur shares six pairs of electrons.
, sulfur shares six pairs of electrons.
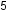 and XeF
and XeF , some of the electrons in the outer shell of the central atom are lone pairs:
, some of the electrons in the outer shell of the central atom are lone pairs: