Table of Contents |
The image below shows the size of a nucleus in an atom about 10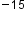 m. If an atom could be expanded to the size of a football stadium, the nucleus would be the size of a single blueberry.
m. If an atom could be expanded to the size of a football stadium, the nucleus would be the size of a single blueberry.

Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. When describing the properties of tiny objects such as atoms, we use appropriately small units of measure, such as the atomic mass unit (amu) and the fundamental unit of charge (e). An amu is exactly 1/12 of the mass of one carbon-12 atom: 1 amu = 1.6605 × 10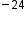 g. The fundamental unit of charge (also called the elementary charge) equals the magnitude of the charge of an electron (e) with e = 1.602 × 10
g. The fundamental unit of charge (also called the elementary charge) equals the magnitude of the charge of an electron (e) with e = 1.602 × 10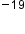 C (C stands for coulomb, which is the standard unit of electric charge).
C (C stands for coulomb, which is the standard unit of electric charge).
A proton has a mass of 1.0073 amu and a charge of 1+. A neutron is a slightly heavier particle with a mass 1.0087 amu and a charge of zero; as its name suggests, it is neutral. The electron has a charge of 1− and is a much lighter particle with a mass of about 0.00055 amu (it would take about 1800 electrons to equal the mass of one proton). The properties of these fundamental particles are summarized below.
| Name | Location | Charge (C) | Unit Charge | Mass (amu) | Mass (g) |
|---|---|---|---|---|---|
| electron | outside nucleus |
−1.602 × 10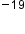
|
1− | 0.00055 |
0.00091 × 10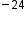
|
| proton | nucleus |
1.602 ×10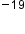
|
1+ | 1.00727 |
1.67262 × 10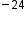
|
| neutron | nucleus | 0 | 0 | 1.00866 |
1.67493 × 10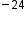
|
The number of protons in the nucleus of an atom is its atomic number (Z). The atomic number determines the identity of the atom. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have.
A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. Therefore, the atomic number also indicates the number of electrons in an atom. The total number of protons and neutrons in an atom is called its mass number (A). The number of neutrons is therefore the difference between the mass number and the atomic number: A – Z = number of neutrons.
Atoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. When the numbers of these subatomic particles are not equal, the atom is electrically charged and is called an ion. The charge of an atom is defined by the formula:
Atoms become charged by gaining or losing electrons. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. Positively charged atoms called cations are formed when an atom loses one or more electrons.
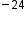 g.
g. 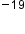 C.
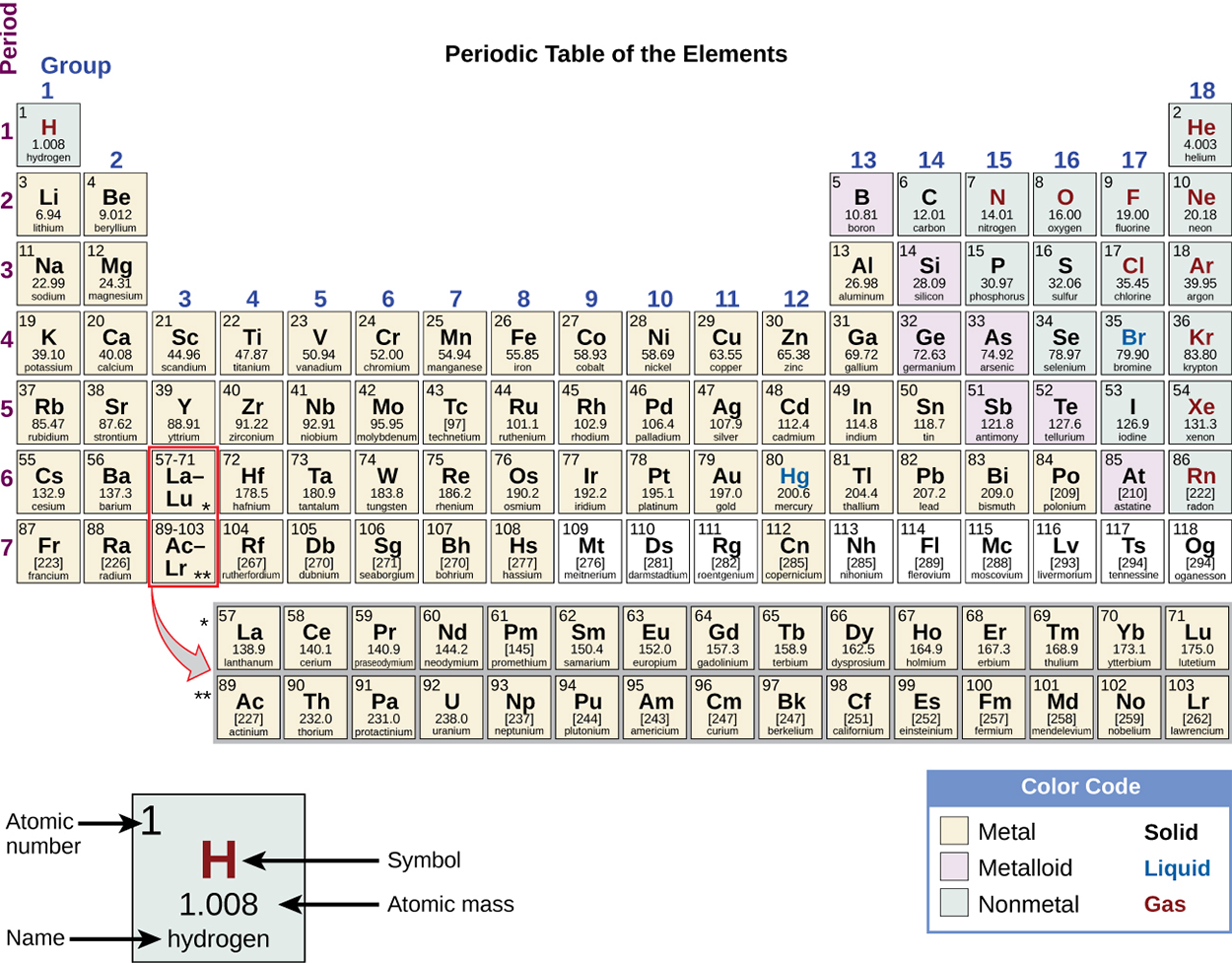
C.As seen in the previous lesson, the periodic table is vital to an understanding of chemistry. The periodic table is made of 118 individual blocks. Each block has 4 pieces of information: symbol, element name, atomic number, and atomic mass. The symbol is the 1 or 2 letter abbreviation for the element. The element name is the name of the element. The atomic number identifies the element and indicates the number of protons. The atomic mass is the mass of the element.
Using the periodic table (seen below) and the formulas from the previous section, the number of subatomic particles in any atom or ion can easily be identified.
Follow this link to WebElements to view an accessible version of the periodic table of elements (Winter, 2021).
EXAMPLE
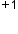 , O, and O
, O, and O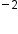 ?
?
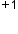 (11 − 10 = 1+) has 10 electrons.
(11 − 10 = 1+) has 10 electrons.
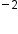 (8 − 10 = 2−) has 10 electrons.
(8 − 10 = 2−) has 10 electrons.
EXAMPLE
A chemical symbol is an abbreviation that we use to indicate an element or an atom of an element. For example, the image below shows the symbol for mercury is Hg. We use the same symbol to indicate one atom of mercury (microscopic domain) or to label a container of many atoms of the element mercury (macroscopic domain).
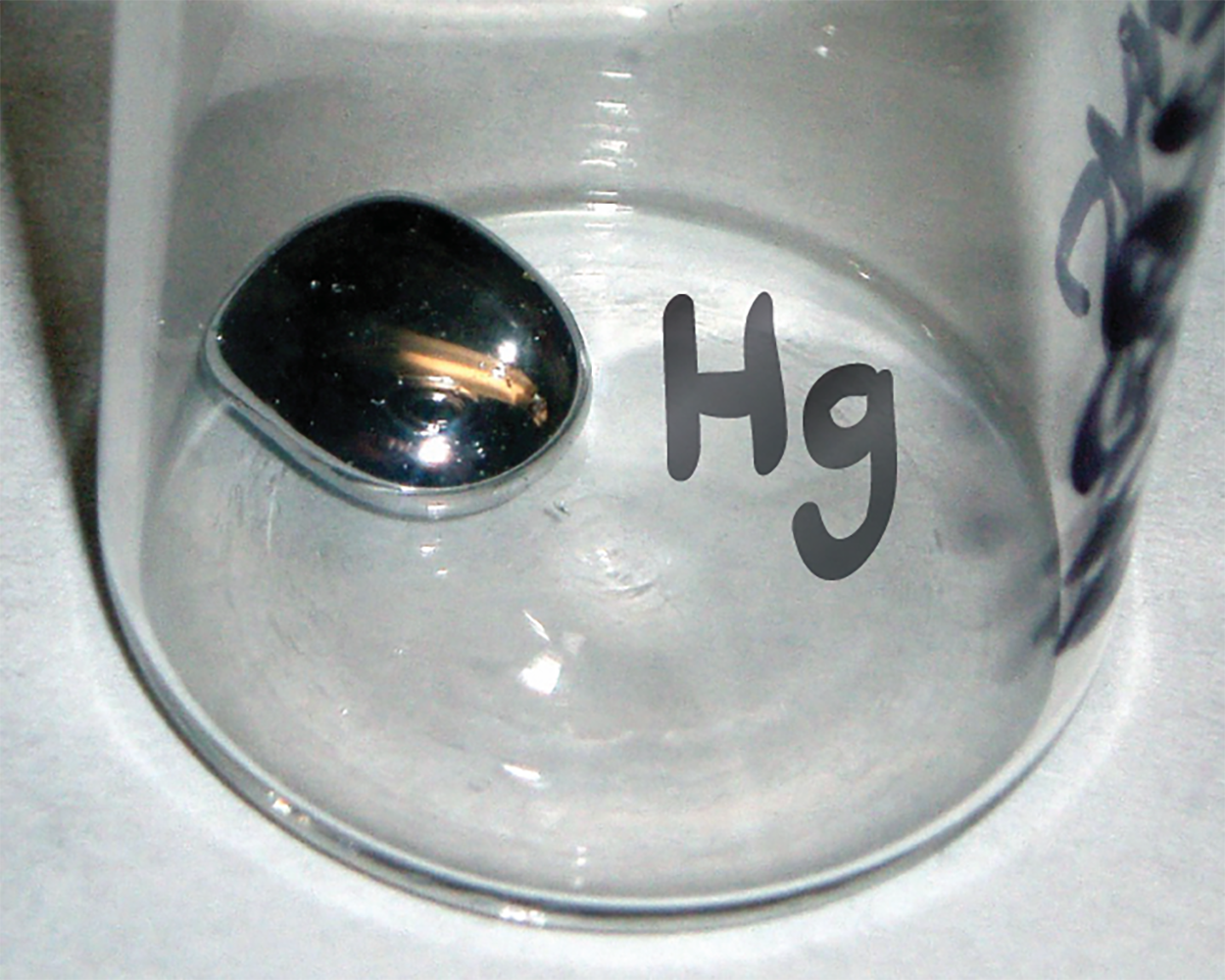
The symbols for several common elements and their atoms are listed in the table below. Some symbols are derived from the common name of the element; others are abbreviations of the name in another language. Most symbols have one or two letters.
To avoid confusion with other notations, only the first letter of a symbol is capitalized. For example, Co is the symbol for the element cobalt, but CO is the notation for the compound carbon monoxide, which contains atoms of the elements carbon (C) and oxygen (O). All known elements and their symbols are found in the periodic table of elements.
| Element | Symbol | Element | Symbol |
|---|---|---|---|
| aluminum | Al | iron | Fe (from ferrum) |
| bromine | Br | lead | Pb (from plumbum) |
| calcium | Ca | magnesium | Mg |
| carbon | C | mercury | Hg (from hydrargyrum) |
| chlorine | Cl | nitrogen | N |
| chromium | Cr | oxygen | O |
| cobalt | Co | potassium | K (from kalium) |
| copper | Cu (from cuprum) | silicon | Si |
| fluorine | F | silver | Ag (from argentum) |
| gold | Au (from aurum) | sodium | Na (from natrium) |
| helium | He | sulfur | S |
| hydrogen | H | tin | Sn (from stannum) |
| iodine | I | zinc | Zn |
IN CONTEXT
Traditionally, the discoverer (or discoverers) of a new element, names the element. However, until the name is recognized by the International Union of Pure and Applied Chemistry (IUPAC), the recommended name of the new element is based on the Latin word(s) for its atomic number.
For example, element 106 was called unnilhexium (Unh), element 107 was called unnilseptium (Uns), and element 108 was called unniloctium (Uno) for several years. These elements are now named after scientists (or occasionally locations); for example, element 106 is now known as seaborgium (Sg) in honor of Glenn Seaborg, a Nobel Prize winner who was active in the discovery of several heavy elements.
The symbol for a specific isotope of any element is written by placing the mass number as a superscript to the left of the element symbol. The atomic number is sometimes written as a subscript preceding the symbol, but since this number defines the element’s identity (as does its symbol), it is often omitted.
Magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26, respectively. These isotopes can be identified as  Mg,
Mg,  Mg, and
Mg, and  Mg. These isotope symbols are read as “element, mass number”. For instance,
Mg. These isotope symbols are read as “element, mass number”. For instance,  Mg is read as “magnesium 24,” and can be written as “magnesium-24” or “Mg-24.” All magnesium atoms have 12 protons in their nucleus. They differ only because a
Mg is read as “magnesium 24,” and can be written as “magnesium-24” or “Mg-24.” All magnesium atoms have 12 protons in their nucleus. They differ only because a  Mg atom has 12 neutrons in its nucleus (number of neutrons = A - Z = 24 - 12 = 12 neutrons), a
Mg atom has 12 neutrons in its nucleus (number of neutrons = A - Z = 24 - 12 = 12 neutrons), a  Mg atom has 13 neutrons, and a
Mg atom has 13 neutrons, and a  Mg has 14 neutrons.
Mg has 14 neutrons.
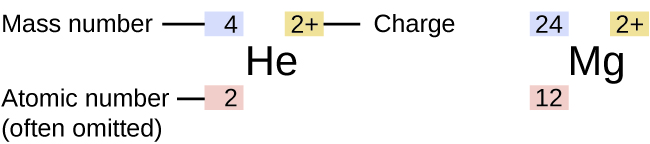
This image shows that the symbol for an atom indicates the element via its usual two-letter symbol, the mass number as a left superscript, the atomic number as a left subscript (sometimes omitted), and the charge as a right superscript.
IN CONTEXT
Note that in addition to standard names and symbols, the isotopes of hydrogen are often referred to using common names and accompanying symbols. Hydrogen-2, symbolizedH, is also called deuterium and sometimes symbolized D. Hydrogen-3, symbolized
H, is also called tritium and sometimes symbolized T.
EXAMPLE
 Br?
Br?

 Br has 35 protons, 35 electrons, and 46 neutrons.
Br has 35 protons, 35 electrons, and 46 neutrons.
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “CHEMISTRY: ATOMS FIRST 2E”. ACCESS FOR FREE AT Chemistry: Atoms First 2e. LICENSE: CREATIVE COMMONS ATTRIBUTION 4.0 INTERNATIONAL
REFERENCES
Winter, M. (2021). The periodic table of the elements. The periodic table of the elements by WebElements. https://webelements.com/.