Table of Contents |
All living things on Earth are formed mostly of carbon compounds. We are “carbon-based” lifeforms. Early chemists regarded substances isolated from organisms (plants and animals) as a different type of matter that could not be synthesized artificially, and these substances were thus known as organic compounds.
The widespread belief called “vitalism” held that organic compounds were formed by a vital force present only in living organisms.
Since then, it has been recognized that organic molecules obey the same natural laws as inorganic substances, and the category of organic compounds has evolved to include both natural and synthetic compounds that contain carbon.
EXAMPLE
Today, organic compounds are key components of plastics, soaps, perfumes, sweeteners, fabrics, pharmaceuticals, and many other substances that we use every day. The value of organic compounds ensures that organic chemistry is an important discipline within the general field of chemistry. In this chapter, we discuss why the element carbon gives rise to a vast number and variety of compounds, how those compounds are classified, and the role of organic compounds in representative biological and industrial settings.The simplest organic compounds contain only the elements carbon and hydrogen and are called hydrocarbons. Even though they are composed of only two types of atoms, there is a wide variety of hydrocarbons because they may consist of varying lengths of chains, branched chains, and rings of carbon atoms, or combinations of these structures.
In addition, hydrocarbons may differ in the types of carbon-carbon bonds present in their molecules. Many hydrocarbons are found in plants, animals, and their fossils; other hydrocarbons have been prepared in the laboratory. We use hydrocarbons every day, mainly as fuels, such as natural gas, acetylene, propane, butane, and the principal components of gasoline, diesel fuel, and heating oil. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms.
Alkanes, or saturated hydrocarbons, contain only single covalent bonds between carbon atoms. Each of the carbon atoms in an alkane is bonded to four other atoms, each of which is either carbon or hydrogen.
EXAMPLE
The Lewis structures and models of methane, ethane, and pentane are shown below.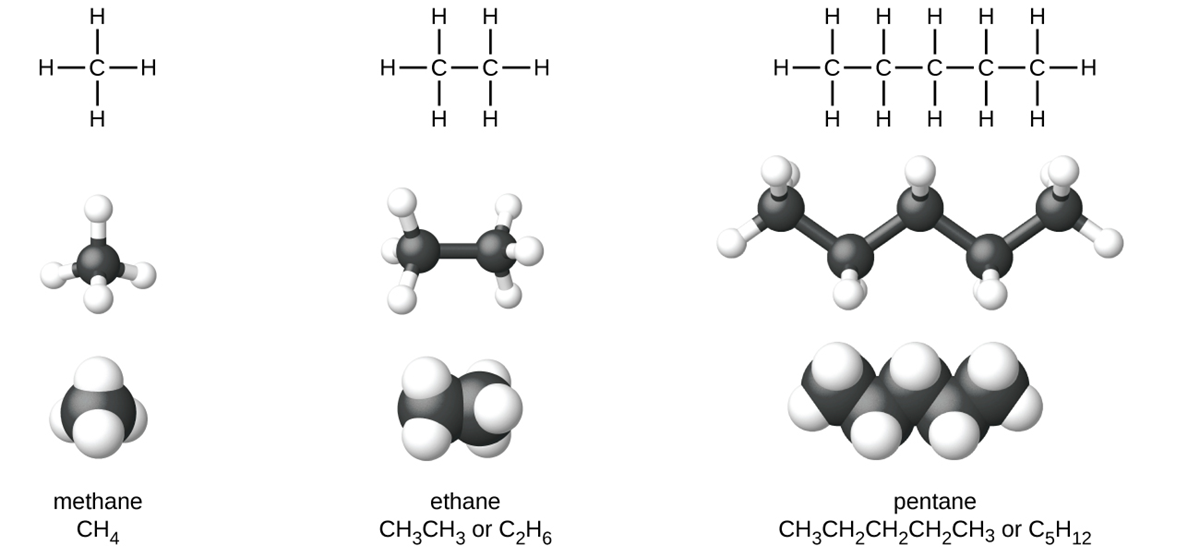
Carbon chains are usually drawn as straight lines in Lewis structures, but one has to remember that Lewis structures are not intended to indicate the geometry of molecules. Notice that the carbon atoms in the structural models (the ball-and-stick and space-filling models) of the pentane molecule do not lie in a straight line. Because of the tetrahedral geometry, the bond angles in carbon chains are close to 109.5°, giving such chains in an alkane a zigzag shape.
The structures of alkanes and other organic molecules may also be represented in a less detailed manner by condensed structural formulas (or simply, condensed formulas). Instead of the usual format for chemical formulas in which each element symbol appears just once (C H
H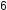 or C
or C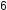 H
H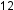 ), a condensed formula is written to suggest the bonding in the molecule. These formulas have the appearance of a Lewis structure from which most or all of the bond symbols have been removed. Condensed structural formulas for ethane (CH
), a condensed formula is written to suggest the bonding in the molecule. These formulas have the appearance of a Lewis structure from which most or all of the bond symbols have been removed. Condensed structural formulas for ethane (CH CH
CH ) and pentane (CH
) and pentane (CH CH
CH CH
CH CH
CH CH
CH ) are shown in the figure above.
) are shown in the figure above.
A common method used by organic chemists to simplify the drawings of larger molecules is to use a skeletal structure (also called a line-angle structure). In this type of structure, carbon atoms are not symbolized with a “C,” but represented by each end of a line or bend in a line. Hydrogen atoms are not drawn if they are attached to a carbon. Other atoms besides carbon and hydrogen are represented by their elemental symbols.
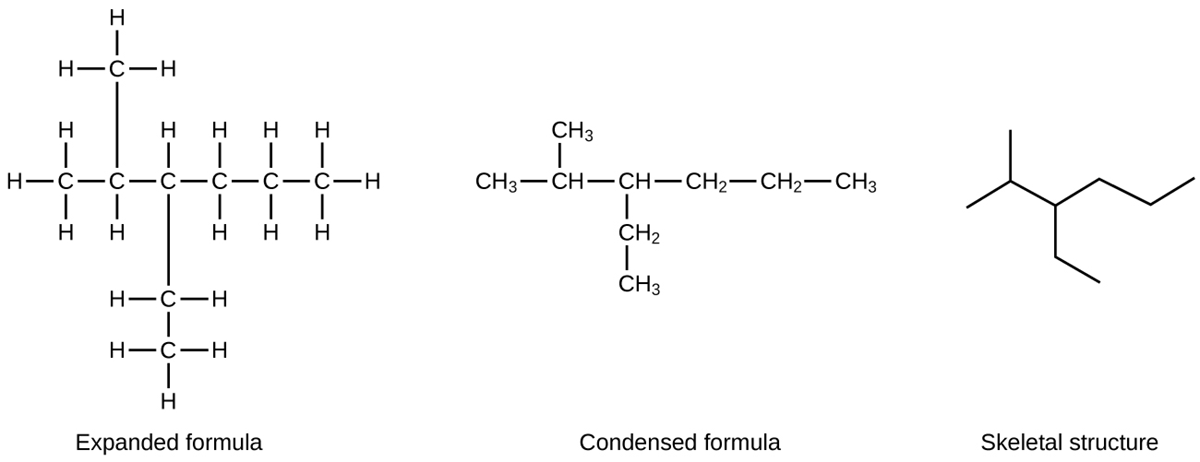
All alkanes are composed of carbon and hydrogen atoms, and have similar bonds, structures, and formulas. The number of carbon atoms present in an alkane has no limit. Greater numbers of atoms in the molecules will lead to stronger intermolecular attractions (dispersion forces) and correspondingly different physical properties of the molecules.
Alkanes are named by the number of carbons in the compound. An alkane with one carbon is methane. A two carbon alkane is ethane. Alkanes with carbons 3-10 are named propane, butane, pentane, hexane, heptane, octane, nonane, and decane, respectively.
Organic compounds that contain one or more double or triple bonds between carbon atoms are described as unsaturated. Unsaturated fats are complex organic molecules with long chains of carbon atoms, which contain at least one double bond between carbon atoms. Unsaturated hydrocarbon molecules that contain one or more double bonds are called alkenes.
Carbon atoms linked by a double bond are bound together by two bonds, one sigma (σ) bond and one pi (π) bond. Double and triple bonds give rise to a different geometry around the carbon atom that participates in them, leading to important differences in molecular shape and properties. The differing geometries are responsible for the different properties of unsaturated versus saturated fats.
EXAMPLE
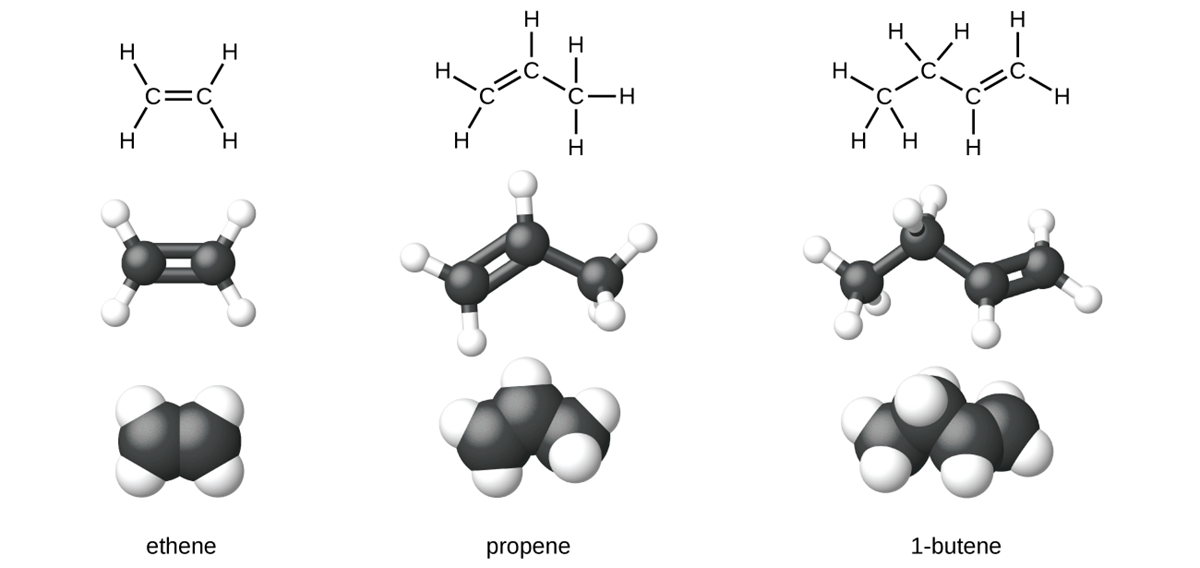
Ethene, C H
H , is the simplest alkene. Each carbon atom in ethene, commonly called ethylene, has a trigonal planar structure. The second member of the series is propene (propylene) and the fourth is butene.
, is the simplest alkene. Each carbon atom in ethene, commonly called ethylene, has a trigonal planar structure. The second member of the series is propene (propylene) and the fourth is butene.
Hydrocarbon molecules with one or more triple bonds are called alkynes; they make up another series of unsaturated hydrocarbons. Two carbon atoms joined by a triple bond are bound together by one sigma (σ) bond and two pi (π) bonds. The carbons involved in the triple bond have bond angles of 180°, giving these types of bonds a linear shape.
EXAMPLE
The simplest member of the alkyne series is ethyne, commonly called acetylene. The Lewis structure for ethyne, a linear molecule, is: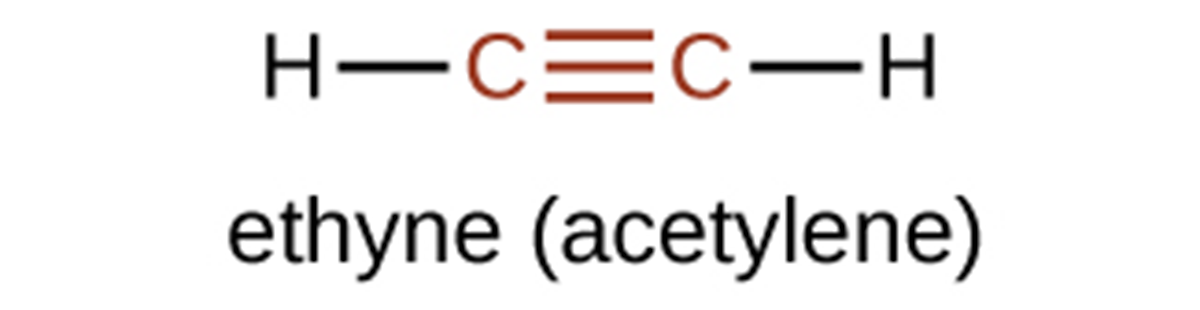
Chemically, the alkynes are similar to the alkenes. Acetylene and the other alkynes also burn readily. An acetylene torch takes advantage of the high heat of combustion for acetylene.
Benzene, C H
H , is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. These compounds contain ring structures. There are two resonance structures for benzene, C
, is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. These compounds contain ring structures. There are two resonance structures for benzene, C H
H , which are shown in the image below:
, which are shown in the image below:
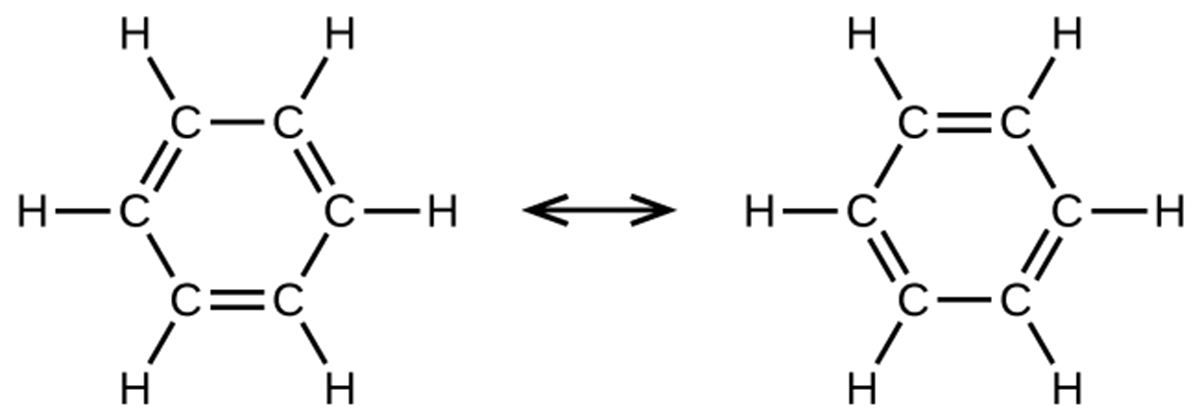
Benzene molecules are flat and planar aromatic hydrocarbon molecules composed of hexagonal rings of carbon atoms with three sets of alternating single and double bonds. Benzene does not, however, exhibit the characteristics typical of an alkene. Each of the six bonds between its carbon atoms is equivalent and exhibits properties that are intermediate between those of a C–C single bond and a C=C double bond.
EXAMPLE
To represent this unique bonding, structural formulas for benzene and its derivatives are typically drawn with single bonds between the carbon atoms and a circle within the ring as shown below: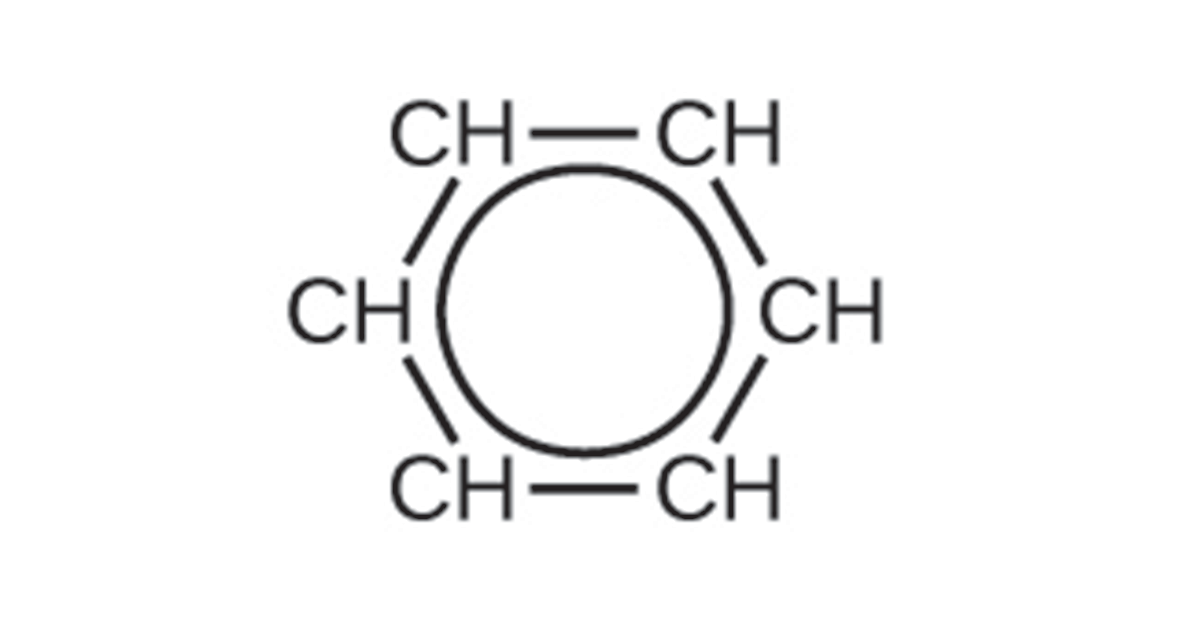
There are many derivatives of benzene. The hydrogen atoms can be replaced by many different substituents. The following are typical examples of substituted benzene derivatives:
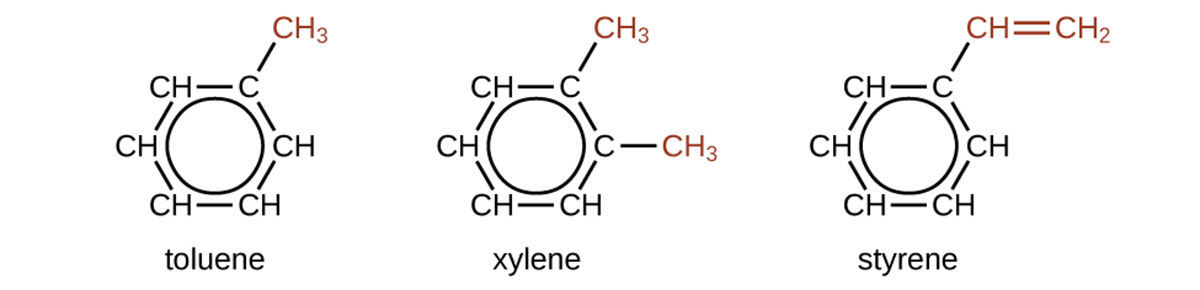
Toluene and xylene are important solvents and raw materials in the chemical industry. Styrene is used to produce polymer polystyrene.
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “CHEMISTRY: ATOMS FIRST 2E”. ACCESS FOR FREE AT Chemistry: Atoms First 2e. LICENSE: CREATIVE COMMONS ATTRIBUTION 4.0 INTERNATIONAL