Table of Contents |
A molecular formula is a representation of a molecule that uses chemical symbols to indicate the types of atoms followed by subscripts to show the number of atoms of each type in the molecule. (A subscript is used only when more than one atom of a given type is present.) Molecular formulas are also used as abbreviations for the names of compounds.
The structural formula for a compound gives the same information as its molecular formula (the types and numbers of atoms in the molecule) but also shows how the atoms are connected in the molecule. The structural formula for methane contains symbols for one C atom and four H atoms, indicating the number of atoms in the molecule. The lines represent bonds that hold the atoms together. (A chemical bond is an attraction between atoms or ions that holds them together in a molecule or a crystal.) We will discuss chemical bonds and see how to predict the arrangement of atoms in a molecule in a later lesson.
For now, simply know that the lines are an indication of how the atoms are connected in a molecule. A ball-and-stick model shows the geometric arrangement of the atoms with atomic sizes not to scale, and a space-filling model shows the relative sizes of the atoms.
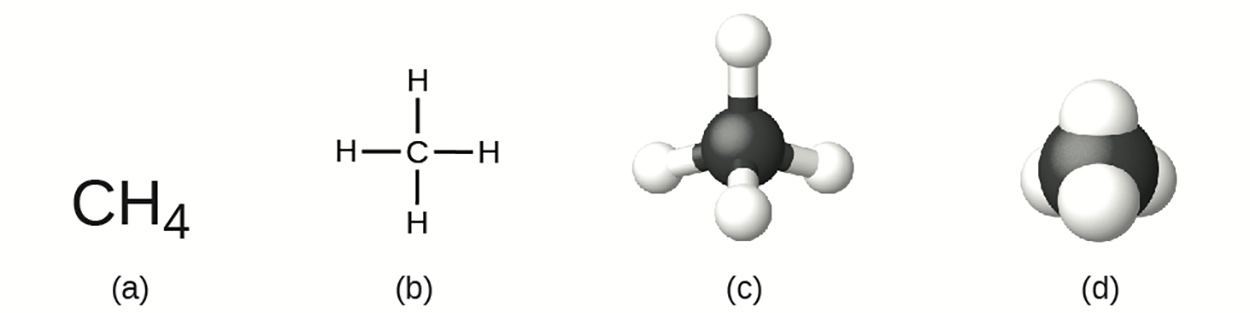
In the images above, a methane molecule is represented as (a) a molecular formula CH , (b) a structural formula, (c) a ball-and-stick model, and (d) a space-filling model. Carbon and hydrogen atoms are represented by black and white spheres, respectively.
, (b) a structural formula, (c) a ball-and-stick model, and (d) a space-filling model. Carbon and hydrogen atoms are represented by black and white spheres, respectively.
Although many elements consist of discrete, individual atoms, some exist as molecules made up of two or more atoms of the element chemically bonded together. For example, most samples of the elements hydrogen, oxygen, and nitrogen are composed of molecules that contain two atoms each (called diatomic molecules) and thus have the molecular formulas H , O
, O , and N
, and N , respectively. Other elements commonly found as diatomic molecules are fluorine (F
, respectively. Other elements commonly found as diatomic molecules are fluorine (F ), chlorine (Cl
), chlorine (Cl ), bromine (Br
), bromine (Br ), and iodine (I
), and iodine (I ).
).
IN CONTEXT
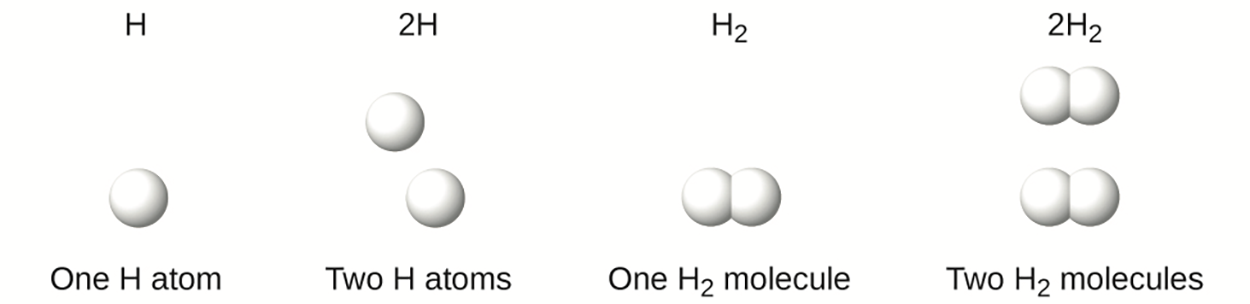
It is important to note that a subscript following a symbol and a number in front of a symbol do not represent the same thing; for example, Hand 2H represent distinctly different species. H
is a molecular formula; it represents a diatomic molecule of hydrogen, consisting of two atoms of the element that are chemically bonded together. The expression 2H, on the other hand, indicates two separate hydrogen atoms that are not combined as a unit. The expression 2H
represents two molecules of diatomic hydrogen.
Compounds are formed when two or more elements chemically combine, resulting in the formation of bonds. For example, hydrogen and oxygen can react to form water, and sodium and chlorine can react to form table salt. We sometimes describe the composition of these compounds with an empirical formula, which indicates the types of atoms present and the simplest whole-number ratio of the number of atoms (or ions) in the compound.
Titanium dioxide (used as a pigment in white paint and in the thick, white, blocking type of sunscreen) has an empirical formula of TiO . This identifies the elements titanium (Ti) and oxygen (O) as the constituents of titanium dioxide, and indicates the presence of twice as many atoms of the element oxygen as atoms of the element titanium.
. This identifies the elements titanium (Ti) and oxygen (O) as the constituents of titanium dioxide, and indicates the presence of twice as many atoms of the element oxygen as atoms of the element titanium.
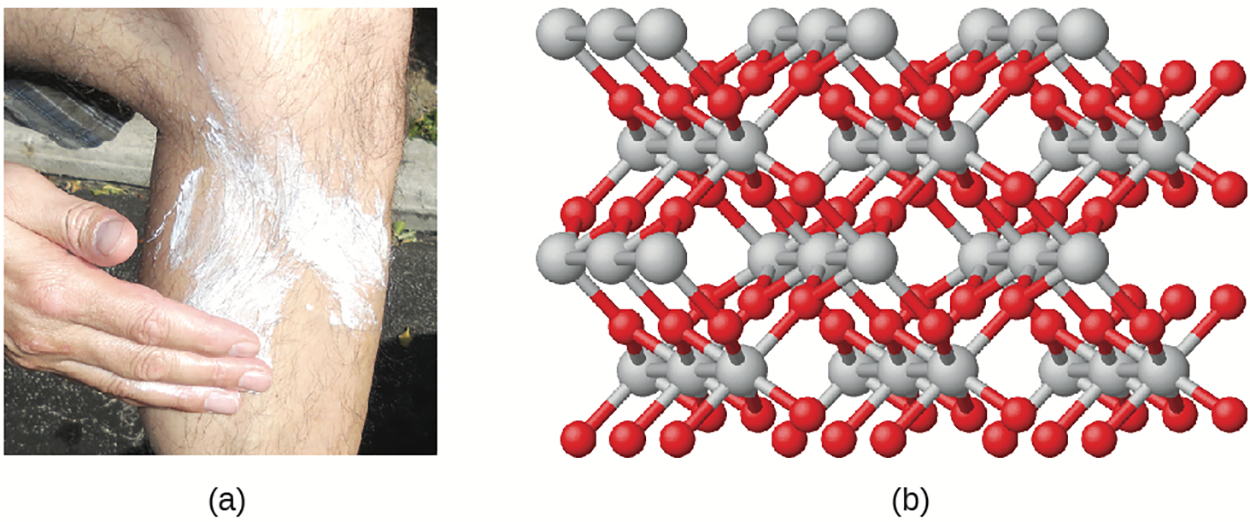
In the above images (a) The white compound titanium dioxide provides effective protection from the sun. (b) A crystal of titanium dioxide, TiO , contains titanium and oxygen in a ratio of 1 to 2. The titanium atoms are gray and the oxygen atoms are red.
, contains titanium and oxygen in a ratio of 1 to 2. The titanium atoms are gray and the oxygen atoms are red.
The empirical formula of benzene was determined experimentally to contain two elements, carbon (C) and hydrogen (H). For every carbon atom in benzene, there is one hydrogen atom; thus, the empirical formula of benzene is CH. An experimental determination of the molecular mass reveals that a molecule of benzene contains six carbon atoms and six hydrogen atoms, so the molecular formula for benzene is C H
H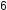 .
.
Benzene, C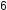 H
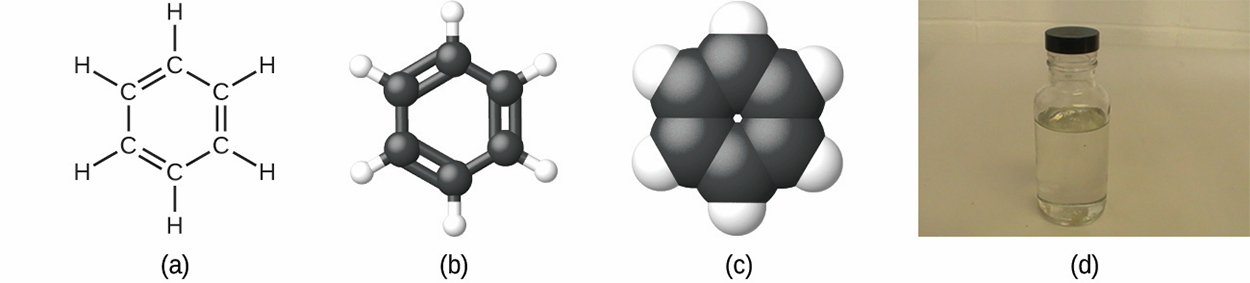
H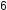 , is produced during oil refining and has many industrial uses. A benzene molecule can be represented in the above image as (a) a structural formula, (b) a ball-and-stick model, and (c) a space-filling model. (d) Benzene is a clear liquid. (credit d: modification of work by Sahar Atwa)
, is produced during oil refining and has many industrial uses. A benzene molecule can be represented in the above image as (a) a structural formula, (b) a ball-and-stick model, and (c) a space-filling model. (d) Benzene is a clear liquid. (credit d: modification of work by Sahar Atwa)
If we know a compound’s formula, we can easily determine the empirical formula.
EXAMPLE
The molecular formula for acetic acid, the component that gives vinegar its sharp taste, is C H
H O
O . This formula indicates that a molecule of acetic acid contains two carbon atoms, four hydrogen atoms, and two oxygen atoms. The ratio of atoms is 2:4:2. Dividing by the lowest common denominator (2) gives the simplest, whole-number ratio of atoms, 1:2:1, so the empirical formula is CH
. This formula indicates that a molecule of acetic acid contains two carbon atoms, four hydrogen atoms, and two oxygen atoms. The ratio of atoms is 2:4:2. Dividing by the lowest common denominator (2) gives the simplest, whole-number ratio of atoms, 1:2:1, so the empirical formula is CH O. Note that a molecular formula is always a whole-number multiple of an empirical formula.
O. Note that a molecular formula is always a whole-number multiple of an empirical formula.
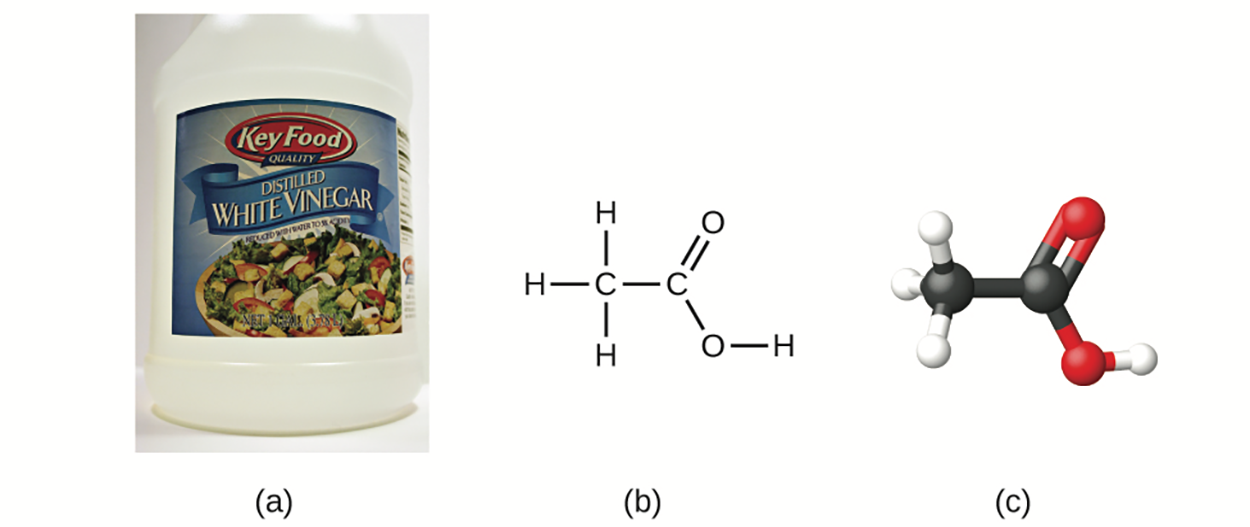
 H
H O
O , which has an empirical formula of CH
, which has an empirical formula of CH O. It can be represented as (b) a structural formula and (c) a ball-and-stick model.
O. It can be represented as (b) a structural formula and (c) a ball-and-stick model.
EXAMPLE
Molecules of glucose (blood sugar) contain 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms. What are the molecular and empirical formulas of glucose?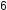 H
H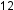 O
O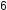 because one molecule actually contains 6 C, 12 H, and 6 O atoms. The simplest whole-number ratio of C to H to O atoms in glucose is 1:2:1, so the empirical formula is CH
because one molecule actually contains 6 C, 12 H, and 6 O atoms. The simplest whole-number ratio of C to H to O atoms in glucose is 1:2:1, so the empirical formula is CH O.
O.
 H
H O
O ? And if so, what would be the structure of its molecules?
? And if so, what would be the structure of its molecules?
If you predict that another compound with the formula C H
H O
O could exist, then you demonstrated good chemical insight and are correct. Two C atoms, four H atoms, and two O atoms can also be arranged to form a methyl formate, which is used in manufacturing, as an insecticide, and for quick-drying finishes. Methyl formate molecules have one of the oxygen atoms between the two carbon atoms, differing from the arrangement in acetic acid molecules.
could exist, then you demonstrated good chemical insight and are correct. Two C atoms, four H atoms, and two O atoms can also be arranged to form a methyl formate, which is used in manufacturing, as an insecticide, and for quick-drying finishes. Methyl formate molecules have one of the oxygen atoms between the two carbon atoms, differing from the arrangement in acetic acid molecules.
Acetic acid and methyl formate are examples of isomers—compounds with the same chemical formula but different molecular structures. Note that this small difference in the arrangement of the atoms has a major effect on their respective chemical properties. You would certainly not want to use a solution of methyl formate as a substitute for a solution of acetic acid (vinegar) when you make salad dressing.
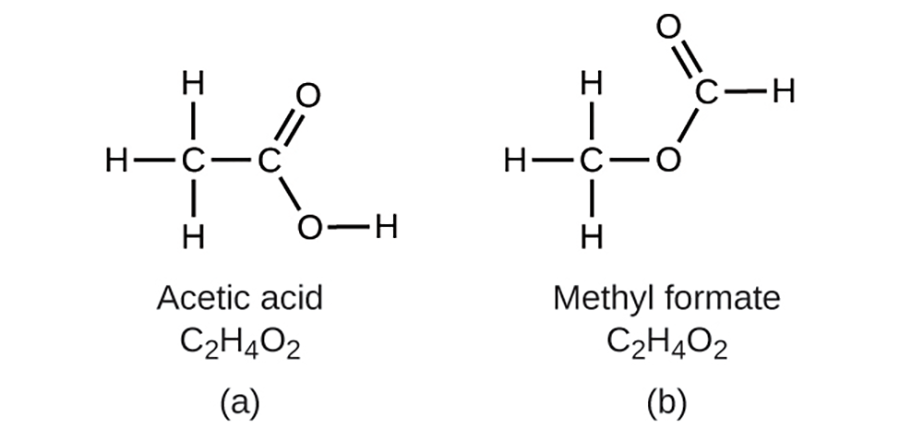
In the above image molecules of (a) acetic acid and methyl formate (b) are structural isomers; they have the same formula (C H
H O
O ) but different structures (and therefore different chemical properties).
) but different structures (and therefore different chemical properties).
Many types of isomers exist. Acetic acid and methyl formate are structural isomers—compounds in which the molecules differ in how the atoms are connected to each other. There are also various types of spatial isomers, in which the relative orientations of the atoms in space can be different.
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “CHEMISTRY: ATOMS FIRST 2E”. ACCESS FOR FREE AT Chemistry: Atoms First 2e. LICENSE: CREATIVE COMMONS ATTRIBUTION 4.0 INTERNATIONAL