Table of Contents |
In the previous lesson, you learned that the kinetic molecular theory can be used to explain the behavior of gases. The kinetic molecular theory may also be used to explain the behavior of solids and liquids. In chemistry, we deal with atoms, molecules, and ions. Many times the laws and theories apply to all three of these equally. So, when it is practical, instead of referring to each individually, we will collectively refer to atoms, molecules, and ions as particles. Additionally, we will use “intermolecular attraction” to refer to attractive forces between the particles of a substance, regardless of whether these particles are molecules, atoms, or ions.
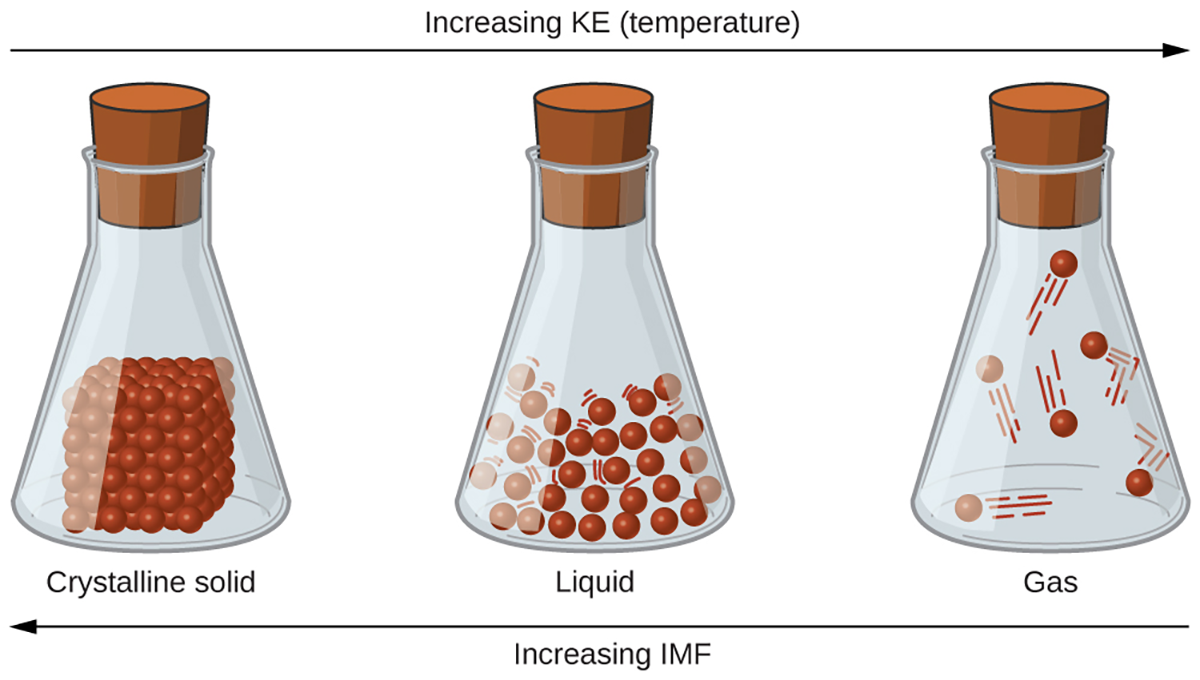
The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the particles (atoms, molecules, or ions) that make up each phase. The phase in which a substance exists depends on the relative extents of its intermolecular forces (IMFs) and the kinetic energies (KE) of its molecules. Intermolecular forces (IMFs) are the various forces of attraction that may exist between the atoms and molecules of a substance due to electrostatic phenomena. Electrostatic refers to static or stationary electrical charges rather than a flow of electricity, which is the definition of current.
The intermolecular forces hold particles close together, whereas the particles’ kinetic energy provides the energy required to overcome the attractive forces and thus increase the distance between particles.
The figure below illustrates how changes in physical state may be induced by changing the temperature, hence, the average kinetic energy, of a given substance. Transitions between solid, liquid, and gaseous states of a substance occur when conditions of temperature or pressure favor the associated changes in intermolecular forces. (Note: The space between particles in the gas phase is much greater than shown).
When gaseous water is cooled sufficiently, the attraction between water molecules will be capable of holding them together when they come into contact with each other; the gas condenses, forming liquid water.
EXAMPLE
Liquid water forms on the outside of a cold glass as the water vapor in the air is cooled by the cold glass (condensation).We can also liquefy many gases by compressing them if the temperature is not too high. The increased pressure brings the molecules of a gas closer together, such that the attractions between the molecules become strong relative to their kinetic energy. Consequently, they form liquids.
EXAMPLE
Butane, C4H10, is the fuel used in disposable lighters and is a gas at standard temperature and pressure. Inside the lighter’s fuel compartment, the butane is compressed to a pressure that results in its condensation to the liquid state, as shown in the following image.
Finally, if the temperature of a liquid becomes sufficiently low, or the pressure on the liquid becomes sufficiently high, the molecules of the liquid no longer have enough kinetic energy to overcome the intermolecular forces between them and a solid will form.
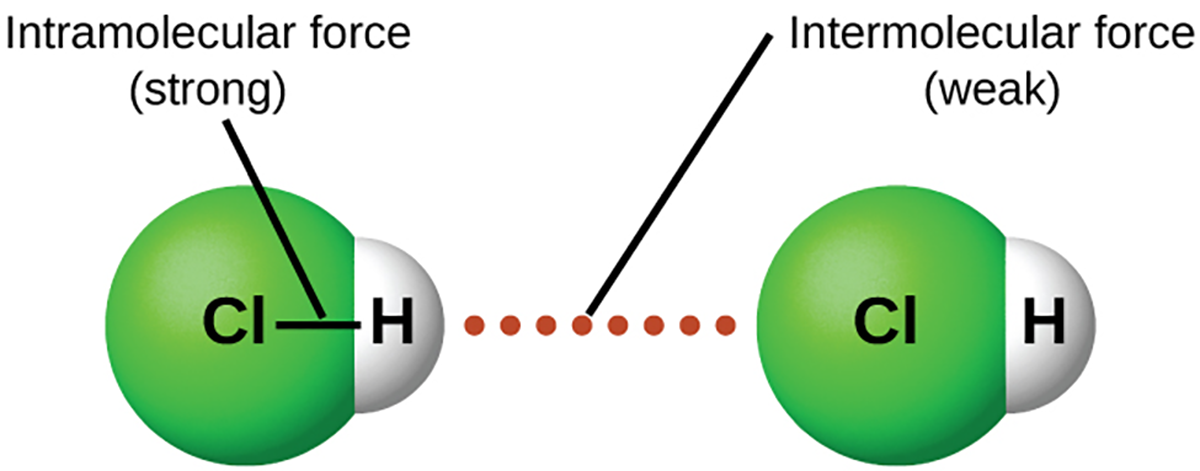
Under appropriate conditions, the attraction between gas molecules will cause them to form liquids or solids. This is due to intermolecular forces, not intramolecular forces. Intramolecular forces are the forces within the molecule that keep the molecule together, such as the bonds between the atoms.
Intermolecular forces are the attractions between molecules, which determine many of the physical properties of a substance. The figure below illustrates these different molecular forces. The strengths of these attractive forces vary widely, though usually, the intermolecular force between small molecules is weak compared to the intramolecular forces that bond atoms together within a molecule.
All of the attractive forces between neutral atoms and molecules are known as van der Waals forces, although they are usually referred to more informally as intermolecular attraction. One of the three van der Waals forces is present in all condensed phases, regardless of the nature of the atoms or molecules composing the substance. This attractive force is called the London dispersion force in honor of German-born American physicist Fritz London who, in 1928, first explained it.
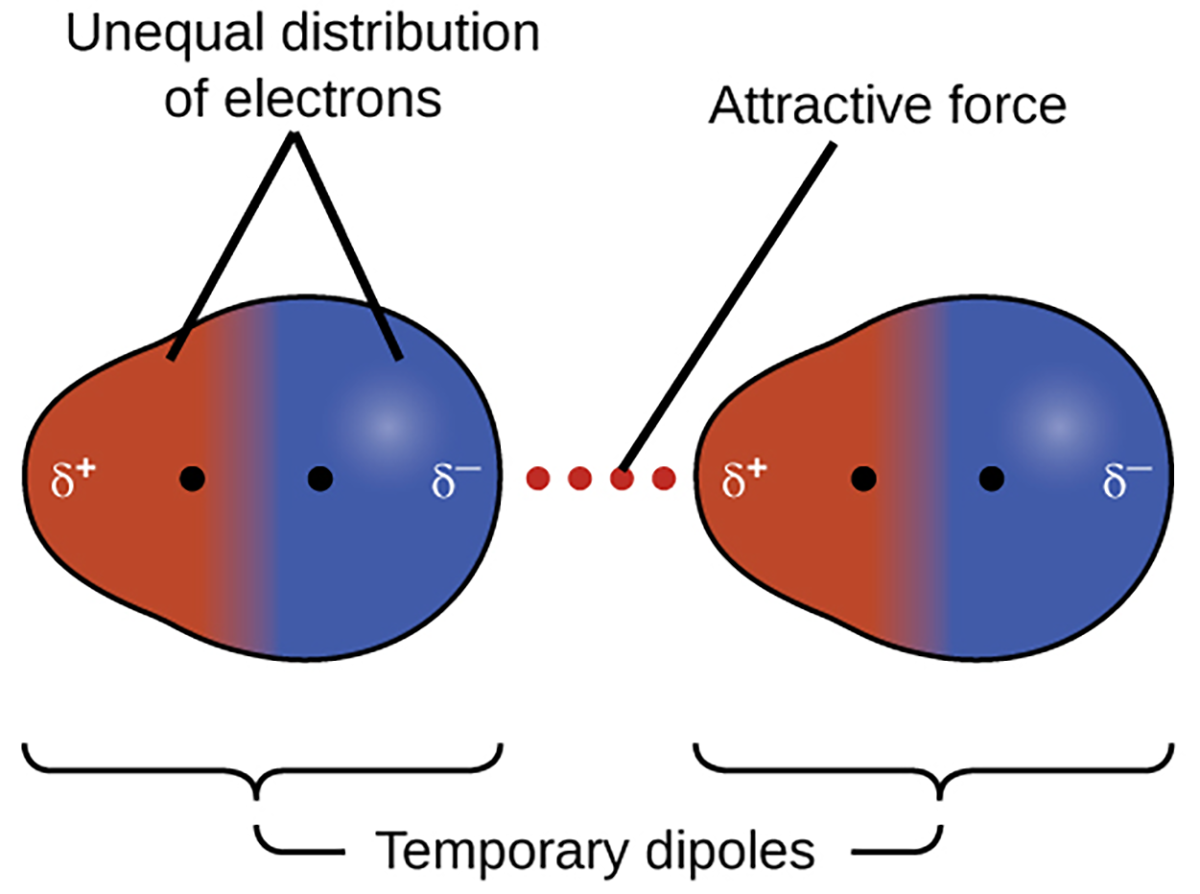
Dispersion forces that develop between atoms in different molecules can attract the two molecules to each other. The forces are relatively weak, however, and become significant only when the molecules are very close. Larger and heavier atoms and molecules exhibit stronger dispersion forces than do smaller and lighter atoms and molecules. F2 and Cl2 are gases at room temperature (reflecting weaker attractive forces); Br2 is a liquid, and I2 is a solid (reflecting stronger attractive forces).
In a larger atom, the valence electrons are, on average, farther from the nuclei than in a smaller atom. Thus, they are less tightly held and can more easily form the temporary dipoles that produce the attraction. The measure of how easy or difficult it is for another electrostatic charge (for example, a nearby ion or polar molecule) to distort a molecule’s charge distribution (its electron cloud) is known as polarizability. A molecule that has a charge cloud that is easily distorted is said to be very polarizable and will have large dispersion forces; one with a charge cloud that is difficult to distort is not very polarizable and will have small dispersion forces.
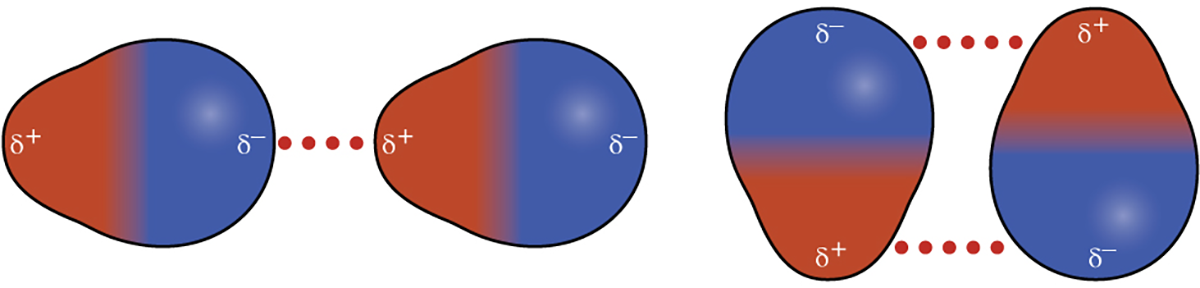
Polar molecules have a partial positive charge on one side and a partial negative charge on the other side of the molecule—a separation of charge called a dipole. In the HCl molecule (a polar molecule), the more electronegative Cl atom bears the partial negative charge, whereas the less electronegative H atom bears the partial positive charge.
An attractive force between HCl molecules results from the attraction between the positive end of one HCl molecule and the negative end of another. This attractive force is called a dipole-dipole attraction—the electrostatic force between the partially positive end of one polar molecule and the partially negative end of another, as seen in the figure below.
The effect of a dipole-dipole attraction is apparent when we compare the properties of HCl molecules to nonpolar F2 molecules. Both HCl and F2 consist of the same number of atoms and have approximately the same molecular mass. At a temperature of 150 K, molecules of both substances would have the same average KE. However, the dipole-dipole attractions between HCl molecules are sufficient to cause them to “stick together” to form a liquid, whereas the relatively weaker dispersion forces between nonpolar F2 molecules are not, and so this substance is gaseous at this temperature.
The higher normal boiling point of HCl (188 K) compared to F2 (85 K) is a reflection of the greater strength of dipole-dipole attractions between HCl molecules, compared to the attractions between nonpolar F2 molecules. We will often use values such as boiling or freezing points, or enthalpies of vaporization or fusion, as indicators of the relative strengths of IMFs of attraction present within different substances.
Nitrosyl fluoride (ONF, molecular mass 49 amu) is a gas at room temperature. Water (H2O, molecular mass 18 amu) is a liquid, even though it has a lower molecular mass. We clearly cannot attribute this difference between the two compounds to dispersion forces. Both molecules have about the same shape and ONF is the heavier and larger molecule. It is, therefore, expected to experience more significant dispersion forces. Additionally, we cannot attribute this difference in boiling points to differences in the dipole moments of the molecules. Both molecules are polar and exhibit comparable dipole moments.
The large difference between the boiling points is due to a particularly strong dipole-dipole attraction that may occur when a molecule contains a hydrogen atom bonded to a fluorine, oxygen, or nitrogen atom (the three most electronegative elements). The very large difference in electronegativity between the H atom and the atom to which it is bonded, combined with the very small size of a H atom and the relatively small sizes of F, O, or N atoms, leads to highly concentrated partial charges with these atoms.
Molecules with F-H, O-H, or N-H moieties are very strongly attracted to similar groups in nearby molecules, producing a particularly strong type of dipole-dipole attraction called a hydrogen bond.
EXAMPLE
examples of hydrogen bonds include HF⋯HF, H2O⋯HOH, and H3N⋯HNH2, in which the hydrogen bonds are denoted by dots.The image below illustrates hydrogen bonding between water molecules:
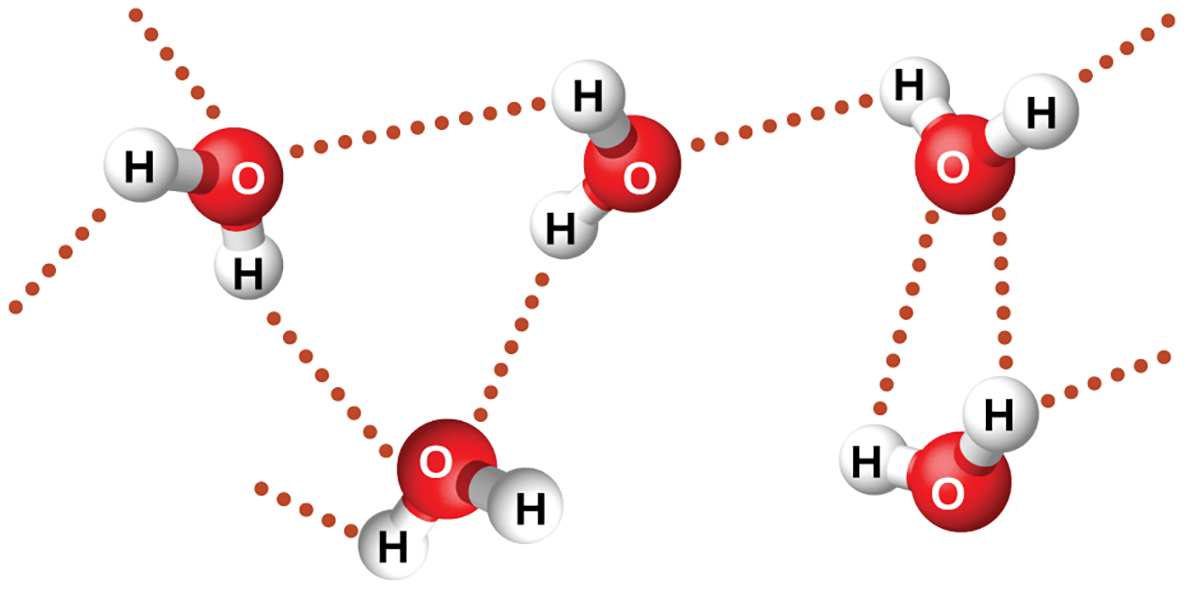
Despite using the word “bond” in the name, hydrogen bonds are intermolecular attractive forces, not intramolecular attractive forces (covalent bonds). Hydrogen bonds are much weaker than covalent bonds, only about 5 to 10% as strong, but are generally much stronger than other dipole-dipole attractions and dispersion forces.
Hydrogen bonds have a pronounced effect on the properties of condensed phases (liquids and solids). For example, consider the trends in boiling points for these compounds of group 15 (NH3, PH3, AsH3, and SbH3), group 16 hydrides (H2O, H2S, H2Se, and H2Te), and group 17 hydrides (HF, HCl, HBr, and HI).
The boiling points of all of these compounds except for NH3, H2O and HF are plotted in the figure below. As we progress down any of these groups, the polarities of the molecules decrease slightly, whereas the sizes of the molecules increase substantially. The effect of increasingly stronger dispersion forces dominates that of increasingly weaker dipole-dipole attractions, and the boiling points are observed to increase steadily as you go down the groups.
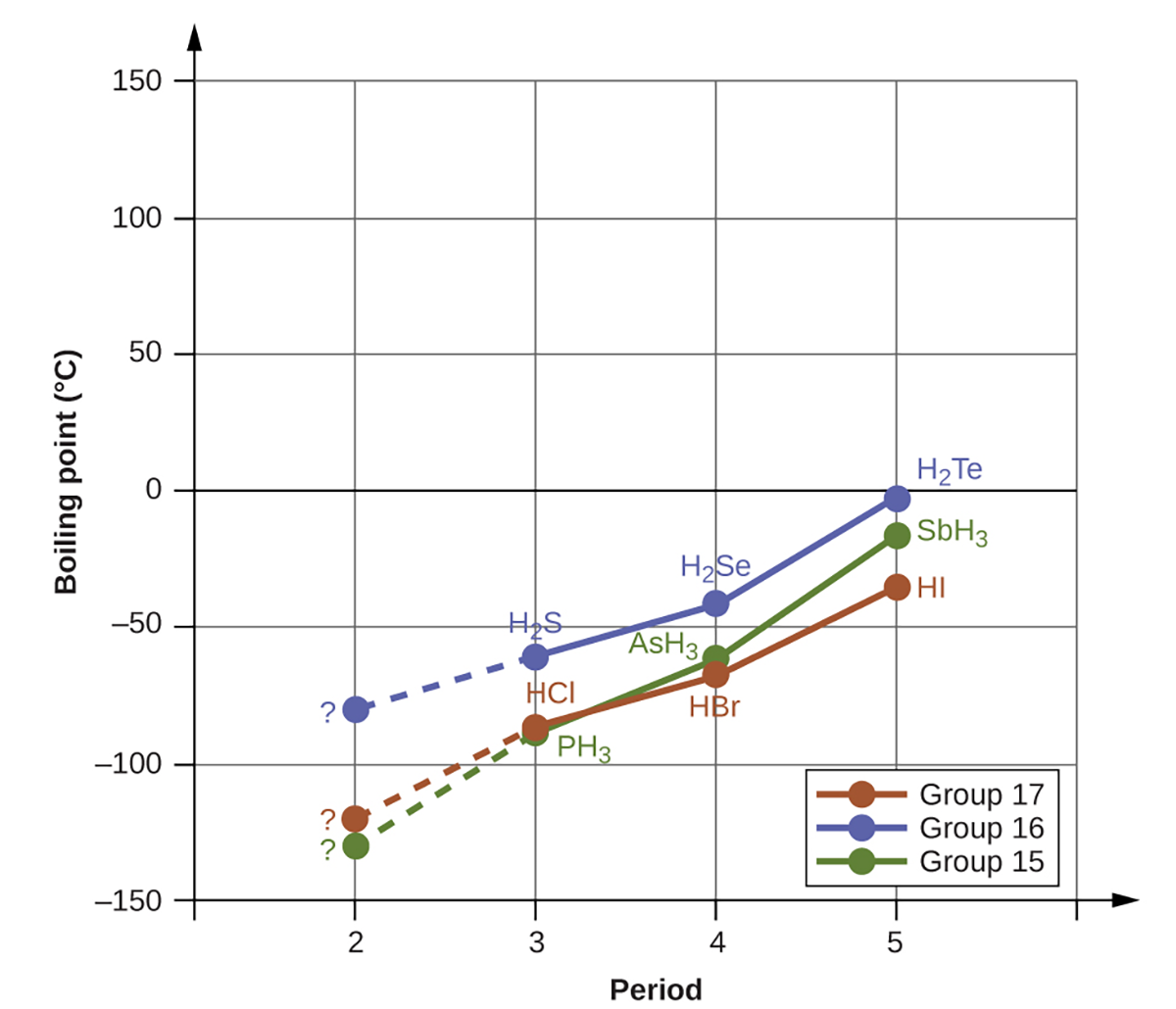
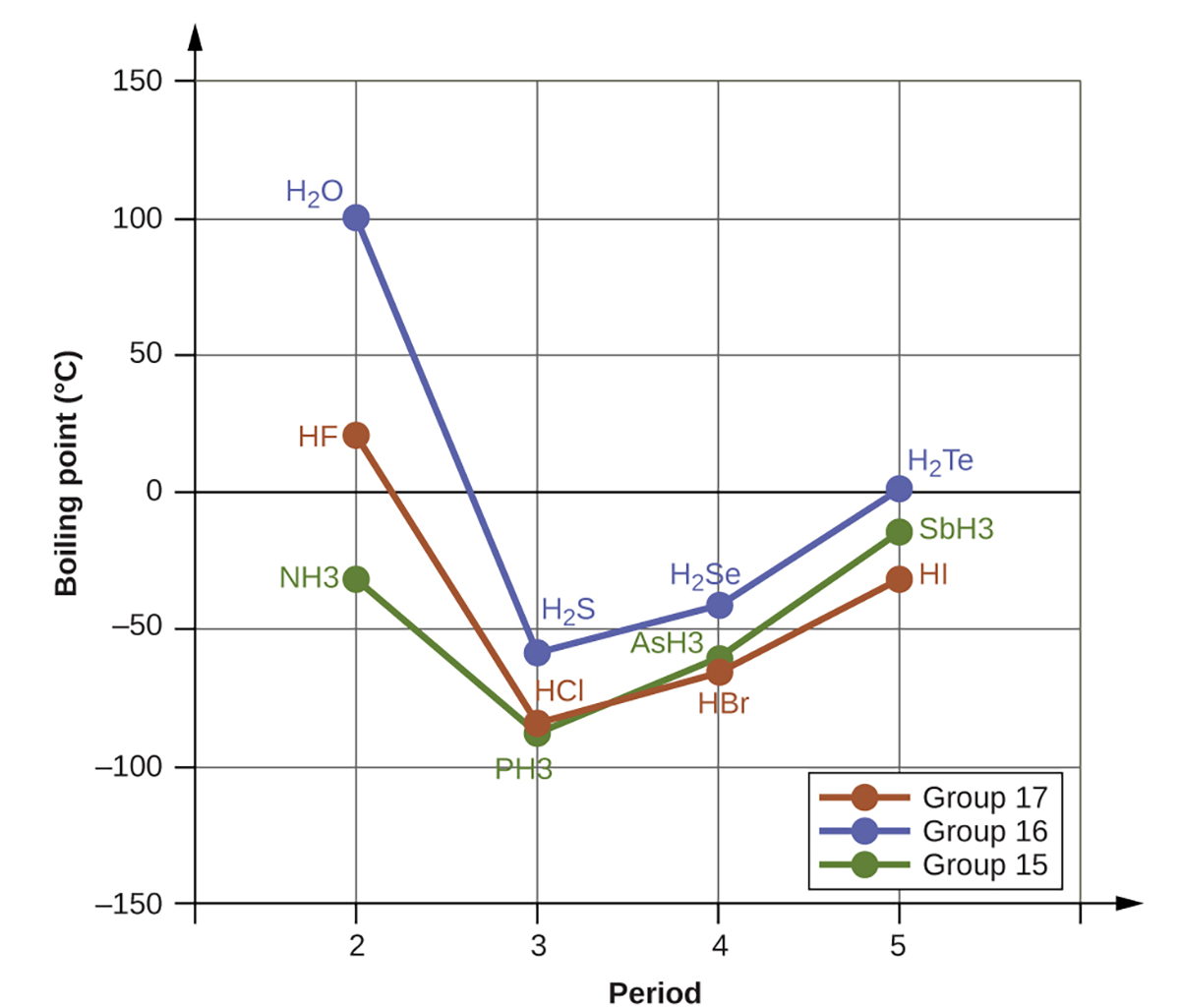
If we use this trend to predict the boiling points for the lightest hydride for each group, we would expect NH3 to boil at about −120°C, H2O to boil at about −80°C, and HF to boil at about −110°C. However, when we measure the boiling points for these compounds, we find that they are dramatically higher than the trends would predict. As shown below, NH3 boils at −28°C (not −120°C), H2O boils at 100 °C (not −80°C), and HF boils at about 19.5°C (not −110°C). The stark contrast between our predictions and reality provides compelling evidence for the strength of hydrogen bonding. NH3, H2O and HF all have hydrogen bonding but the other 9 compounds (graphed above) do not have hydrogen bonding.
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “CHEMISTRY: ATOMS FIRST 2E”. ACCESS FOR FREE AT Chemistry: Atoms First 2e. LICENSE: CREATIVE COMMONS ATTRIBUTION 4.0 INTERNATIONAL