Table of Contents |
Many of our tutorials have addressed control methods for quality. It is also important to address how companies determine quality in an objective way by enlisting outside organizations or independent departments within the organization to run inspections or perform audits (official and thorough inspections). Continuous improvement relies on different types of inspections to ensure ongoing enhancement of processes and products. The key difference between the methods described here and those discussed previously is that inspections and audits suggest an outside, objective party is involved, such as a contractor hired to do a fair assessment or a department within the organization that can operate independently. Inspections and audits will usually be delivered as a Quality Improvement Plan (QIP), a report of findings with “grades” and recommendations.
EXAMPLE
Hospitals and clinics will have regular inspections by state or civic units that provide licensure to operate. Because these inspections can be costly in terms of revenue and reputation, the healthcare provider is likely to have voluntary inspections done by other organizations before the mandatory inspections so they can solve any problems in advance. Similar inspections are involved across several industries, from daycare providers to restaurants.First, let’s look at the different types of inspections and audits that can occur.
Pre-production inspections (PPI) occur before production begins. The quality of raw materials is inspected. This type of inspection can save a lot of time in the long run.
EXAMPLE
Suppose a company is making sweatshirts from recycled materials. They discover later that a lot of people find the shirts itchy and uncomfortable due to allergens in the materials. Had pre-inspection been done, it would’ve saved time and effort by identifying this issue before those items went into production.During production inspection (DPI or DUPRO) is when checks are done mid-process. There is likely to be an initial production check of the first few items produced to verify the initial products meet quality standards.
Inline quality control (IQC) inspections are done at critical points in the process. For example, there might be checks after a component has been built, then another when the component has been welded to another part.
Sample checking is the process of choosing representative items to check for quality. This process involves identifying the total number of units to inspect, and which batch to inspect. The units from the lot will be randomly selected either using systematic sampling or stratified sampling. Systematic sampling refers to selection of units at regular intervals, such as every tenth item. Stratified sampling means dividing the lot into subgroups and sampling from each group to ensure representation.
The types of tests done for sample checking might include visual inspection, where the quality control person looks for visible defects such as scratches, dents, or incorrect color. Dimensional inspections refer to the measurement of critical dimensions to ensure they meet specified tolerances.
Functional testing means to ensure the product meets specifications and operates the way it is intended. Material testing means conducting tests to verify the properties of the product, such as strength, meet the specifications.

Gordon’s Bicycle Company uses a variety of inspection methods. They inspect the materials and pre-made components as they arrive.
They check the digital components before they are installed (IQC) and do sample checking and functional testing by taking every 100th bicycle for a ride with stress testing. Because Gordon is aware that his ownership of the company might skew his inspections, he uses a third-party auditing company to run these tests.
Many companies will opt to perform supplier site inspections to reduce supply chain risk. The first reason for this is to ensure consistency in quality and ensure the raw materials and products meet the standards agreed upon by both companies. This also allows the organization to ensure the suppliers' manufacturing processes are capable of producing consistent quality.
Supplier site inspections can also ensure regulatory compliance, to ensure the supplier adheres to regulatory or ISO standards. Inspections can help manage a brand's reputation in quality and also factory and facility working conditions for workers.
EXAMPLE
Foxconn, the iPhone factory located in China, was found to have horrific working conditions, which created negative public relations for the company. In response, Apple increased the frequency and rigidity of audits at the factory and encourages the Fair Labor Association to conduct audits too (Labor News, 2023).Ultimately, a supplier site inspection can provide risk mitigation for a company. By identifying potential issues through these inspections, they can identify and address potential quality issues and ensure the supply chain isn’t disrupted due to poor-quality products.
Many organizations will also perform supplier quality audits. A quality audit assists a company in identifying, preventing, and remedying problems in a supplier's process or product quality. Remember, the goal is to have strong partnerships with suppliers, so by working together, the two organizations can address any issues with the quality of raw materials or supplied goods.
The following steps are written for the team doing the audit, whether a company inspecting a supplier or an external organization hired to do an audit. Either way, an audit has six main steps: preparation and planning, conducting the audit, evaluation and analysis, reporting, follow-up and corrective actions, and continuous improvement. Let’s look at these steps in turn (QIMA, 2024).
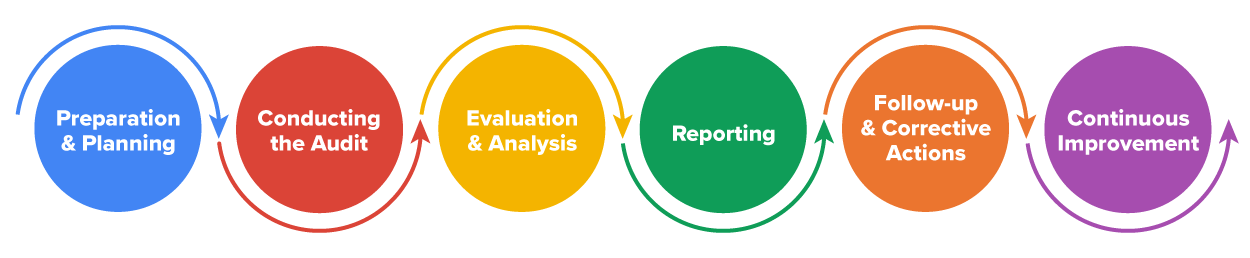
First, a company must prepare for the audit. Remember, for continuous improvement, these audits should be performed systematically to ensure compliance with quality, regulatory, and contractual requirements. Here are the steps to take in the preparation and planning phase of an audit:
The next phase is to conduct the audit. Here are the usual steps that occur during the audit phase.
After the audit is conducted, the evaluation and analysis of the audit will occur. Here are the steps to take in that phase.
The next step is to draft the audit report and provide specific recommendations. Just as it is at every phase, it is important to remember a strong relationship between the company and auditor is important, so the report should be approached as a collaborative, problem-solving effort.
The final step, continuous improvement, involves the following:
As you may recall, continuous improvement is the process that involves incremental (small) changes to systems, processes, and products to continually see better results. As you’ve learned in this tutorial, inspections and audits are two methods to determine causes of quality issues so they can be addressed. But, continuous improvement is an ongoing process.
Organizations, based on inspections, audits, observations, and customer feedback, will normally follow a continuous improvement process by following these steps:
Source: This tutorial has been adapted from Saylor Academy and NSCC “Operations Management”. Access for free at https://pressbooks.nscc.ca/operationsmanagement2/. License: Creative Commons Attribution 4.0 International.
REFERENCES
Labor News (2023, November 10). Investigation of an Apple Supplier: Chengdu Foxconn Report in 2023. China Labor Watch. chinalaborwatch.org/investigation-of-an-apple-supplier-chengdu-foxconn-report-in-2023/
QIMA (2024, July 3). How to conduct a supplier audit. QIMA. blog.qima.com/supplier-audits/how-to-conduct-a-supplier-audit