Table of Contents |
The smallest, most fundamental material components of the human body are basic chemical elements. In fact, chemicals called nucleotide bases (or nucleotides) are the foundation of the genetic code, which has the instructions on how to build and maintain the human body from conception through old age. There are about three billion nucleotide base pairs in human DNA.
Human chemistry includes organic molecules (which are carbon based), biochemicals (which are produced by the body), and other elements. In fact, life cannot exist without many of the elements that are part of the Earth. All of the elements that contribute to chemical reactions, to the transformation of energy, and to electrical activity and muscle contraction originated in stars. These elements include phosphorus, carbon, sodium, and calcium, to name a few. These elements, in turn, can form both the inorganic and organic chemical compounds important to life, including, for example, water, glucose (sugar), and proteins.
The following sections examine inorganic and organic compounds that are essential to human life.
An inorganic compound is a substance that does not contain both carbon and hydrogen. Many inorganic compounds do contain hydrogen atoms, such as water (H₂O) and the hydrochloric acid (HCl) produced by your stomach. In contrast, only a handful of inorganic compounds contain carbon atoms. Carbon dioxide (CO₂) is one of the few examples.
Water (H₂O) is contained both within the cells and between the cells that make up tissues and organs, and it has several unique properties that are indispensable to human functioning.
Water is a major component of many of the body’s lubricating fluids. These fluids function like oil in the hinge of a door—decreasing friction to increase function and the life of the door (organ or tissue).
Water can absorb large amounts of energy. This makes water an excellent heat sink, which is a substance or object that absorbs and dissipates heat but does not experience a corresponding increase in temperature. In the body, water absorbs the heat generated by chemical reactions without greatly increasing in temperature.
Moreover, when the environmental temperature soars, the water stored in the body helps keep the body cool. This cooling effect happens as warm blood from the body’s core flows to the blood vessels just under the skin. At the same time, sweat glands release warm water in sweat. As the water evaporates into the air, it carries away heat. Then, the cooler blood near the skin circulates back to the body's core.
For cells in the body to survive, they must be kept moist in a water-based liquid called a solution. In chemistry, a solution consists of two substances, a solvent and a solute. A solvent is the substance, present in a greater amount, that does the dissolving, whereas a solute, present in a smaller amount, is the substance being dissolved. When a solvent and solute are mixed so that all molecules are evenly distributed, they form a solution.
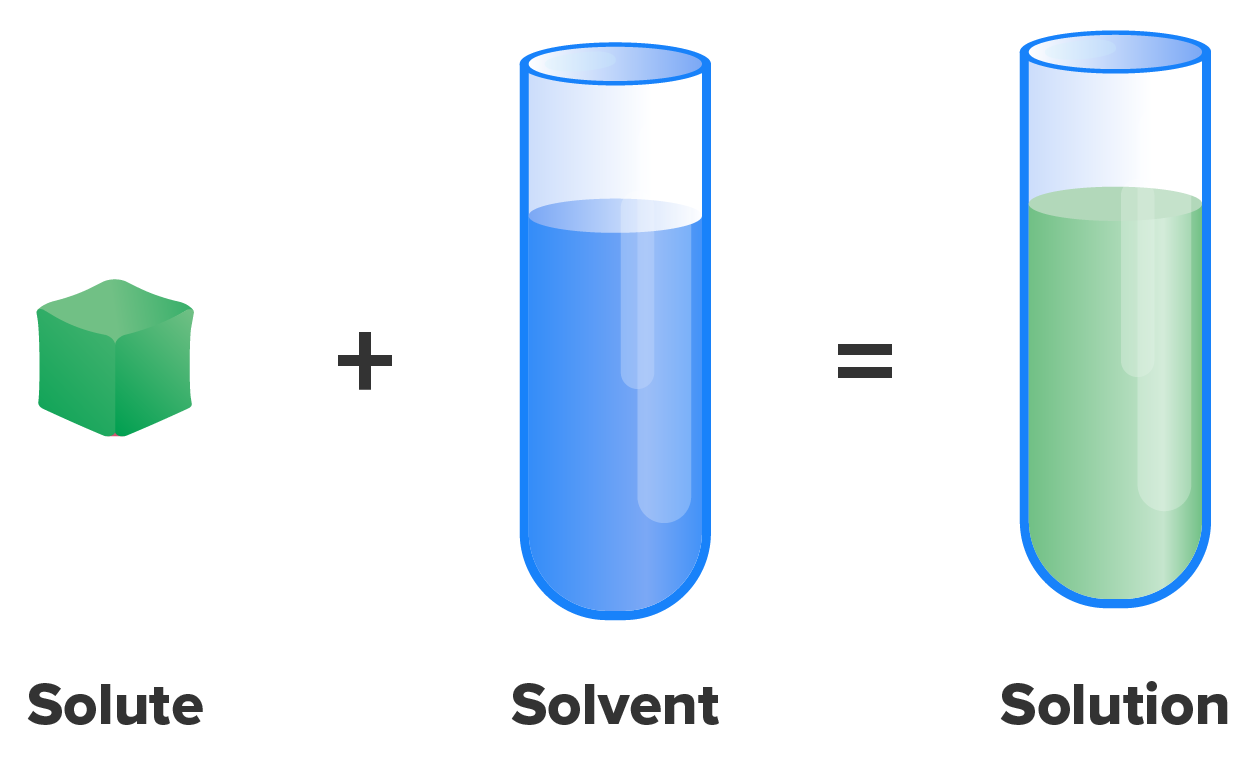
Substances of all states of matter (solid, liquid, gas) can form solutions—nickel mixed into gold to form white gold in jewelry, coffee bean grounds mixed into water to form coffee, oxygen mixed into nitrogen to form the compressed air scuba divers breathe. However, the solutions in the human body are primarily formed with liquid water as the solvent. Water is commonly considered the “universal solvent,” and it is believed that life cannot exist without water because of this. Water is certainly the most abundant solvent in the body, dissolving a wide range of molecules and compounds.
The hydrogen and oxygen atoms within water molecules form polar covalent bonds, which are a type of bond in which electrons are pulled toward one atom and away from another. This results in slightly positive and slightly negative charged regions of the molecule. Water molecules are able to dissolve compounds that are hydrophilic, or “water-loving.” Sugar dissolves well in water due to its polar bonds, making it hydrophilic. When a cube of sugar is dropped into a cup of coffee, the sugar dissolves.
Nonpolar molecules have electrons that are shared equally between atoms and therefore have no regions with partial charges as in polar molecules. These molecules do not readily dissolve in water and are called hydrophobic, or “water-fearing.” Oil is hydrophobic, which is why oil and water do not mix. Even when vigorously shaken together, oil and water will naturally separate from one another due to the hydrophobic nature of oil.

Water as a solvent provides several important features to the body.
Salt is a substance that, when dissolved in water, dissociates into ions other than H⁺ (hydrogen ions) or OH⁻ (hydroxide ions). This fact is important in distinguishing salts from acids and bases.
A typical salt, sodium chloride (or NaCl), dissociates completely in water, as shown in the image below. The partial positive end of the water molecule (the hydrogen atom, H) attracts the negative chloride ion (Cl⁻). The partial negative end of the water molecule (the oxygen atom, O) attracts the positive sodium ion (Na⁺). These attractions overcome the attractive forces of the ionic bond and separate the ions from one another. These ions are electrolytes; they are capable of conducting an electrical current in a solution. This property is critical to the function of ions in transmitting nerve impulses and promoting muscle contraction.
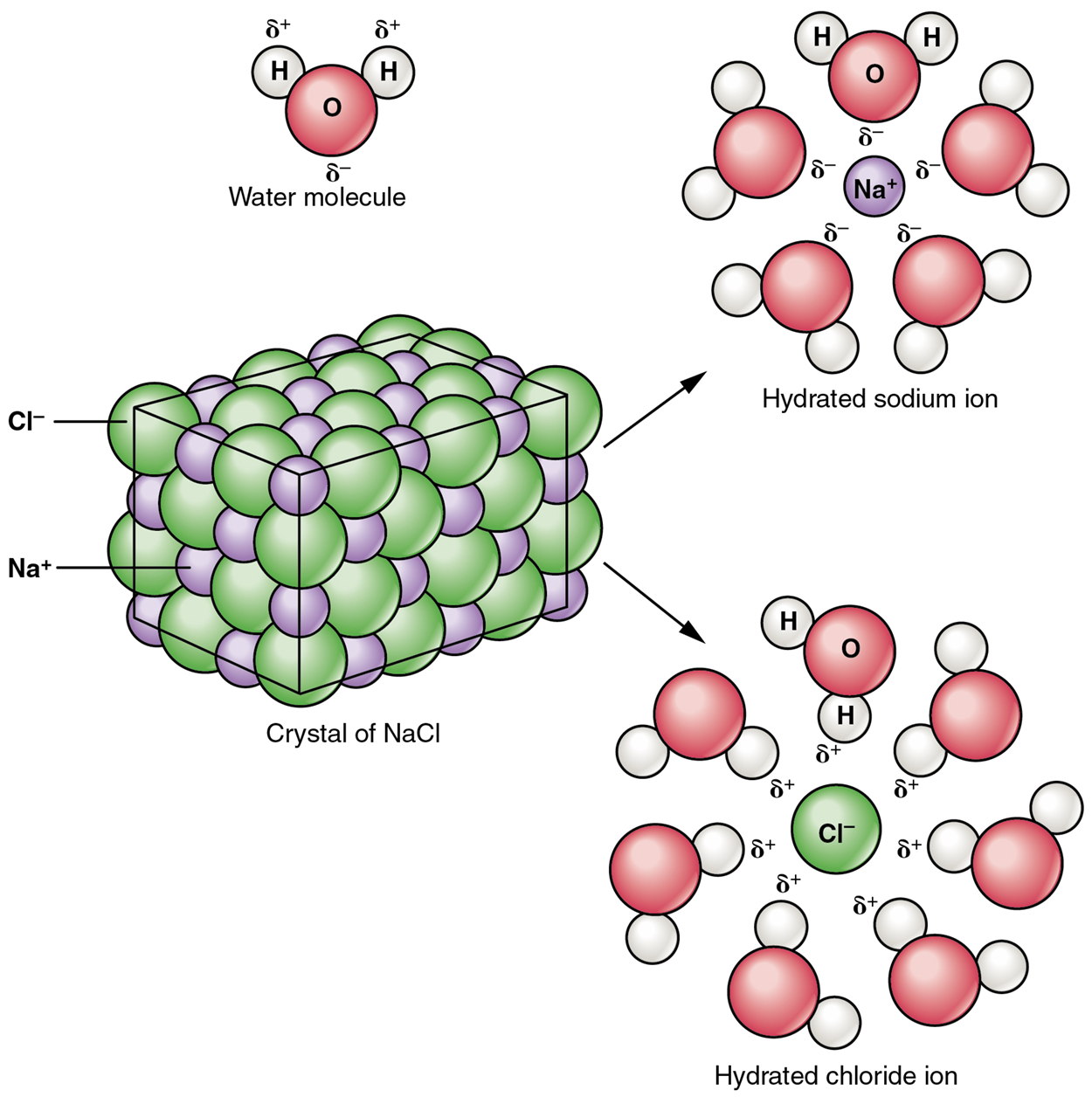
Many other salts are also important in the body.
EXAMPLE
Bile salts produced by the liver help break apart dietary fats, and calcium phosphate salts form the mineral portion of teeth and bones.Acids and bases, like salts, dissociate in water into electrolytes. Acids and bases can very much change the properties of the solutions in which they are dissolved.
An acid is a substance that releases hydrogen ions (H⁺) in solution. Because an atom of hydrogen has just one proton and one electron, a hydrogen ion, having donated its only electron, is simply a proton. This solitary proton is highly likely to participate in chemical reactions. Acids, or acidic substances, are graded based on how many of their available hydrogen ions they will release in the solution. Strong acids are compounds that release all of their H⁺ in solution; that is, they ionize completely. Weak acids do not ionize completely; that is, some of their hydrogen ions remain bonded within a compound in solution.
EXAMPLE
Hydrochloric acid (HCl), which is released from cells in the lining of the stomach, is a strong acid because it releases all of its H⁺ into the stomach’s watery environment. This strong acid aids in digestion and kills ingested microbes. Vinegar, or acetic acid, is an example of a weak acid and is used commonly in cooking. Weak acids release a much smaller share of their H⁺ ions.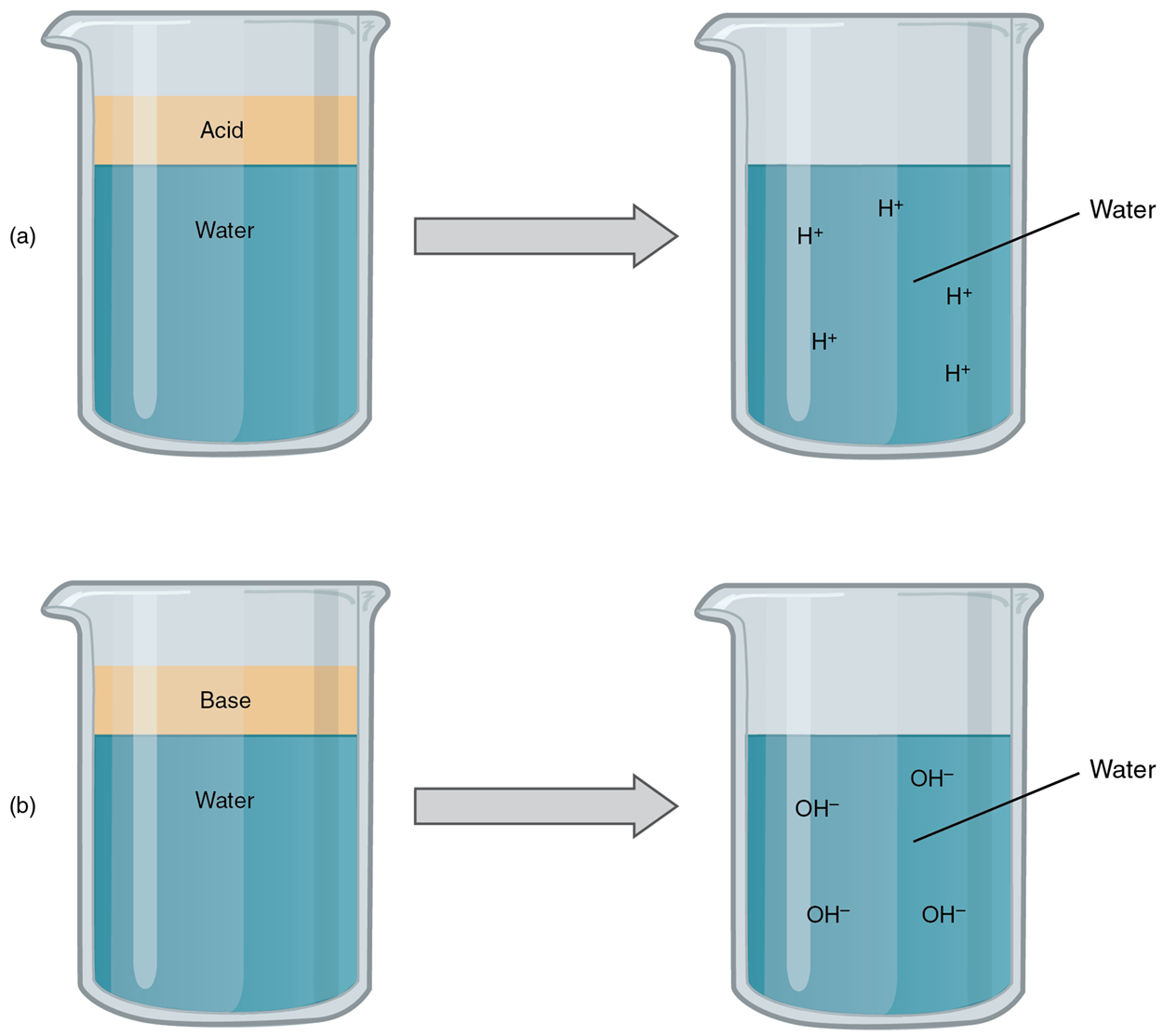
A base is a substance that releases hydroxyl ions (OH⁻) in a solution or one that accepts H⁺ already present in a solution. The hydroxyl ions (also known as hydroxide ions) or other basic substances combine with H⁺ present to form a water molecule, thereby removing a free H⁺ and reducing the solution’s acidity. Like acids, bases are graded based on the proportion of hydroxyl ions they release or hydrogen ions they accept. Strong bases release most or all of their hydroxyl ions; weak bases release only some hydroxyl ions or absorb only a few H⁺. Bases that dissolve in water are also known as alkali or alkaline substances. Common household alkaline substances include baking soda, soap, ammonia, and bleach.
EXAMPLE
As food mixed with hydrochloric acid (HCl) leaves the stomach, it enters the small intestine. Here, if it were not for the production of bicarbonate (HCO₃⁻), a weak base that attracts H⁺, the food would burn into or through the lining of the small intestine. Bicarbonate accepts some of the H⁺ ions, thereby reducing the acidity of the solution and preserving the lining of the digestive tract.The relative strength of an acid (acidity) or alkali base (alkalinity) in a solution can be indicated by its pH. A solution’s pH is a measure of the hydrogen ion (H⁺) concentration of the solution and can range from 0 to 14. A solution with a pH of 7 is considered neutral—neither acidic nor basic. Pure water has a pH of 7. The lower the number below 7, the more acidic the solution, or the greater the concentration of H⁺. The higher the number above 7, the more basic (alkaline) the solution, or the lower the concentration of H⁺.
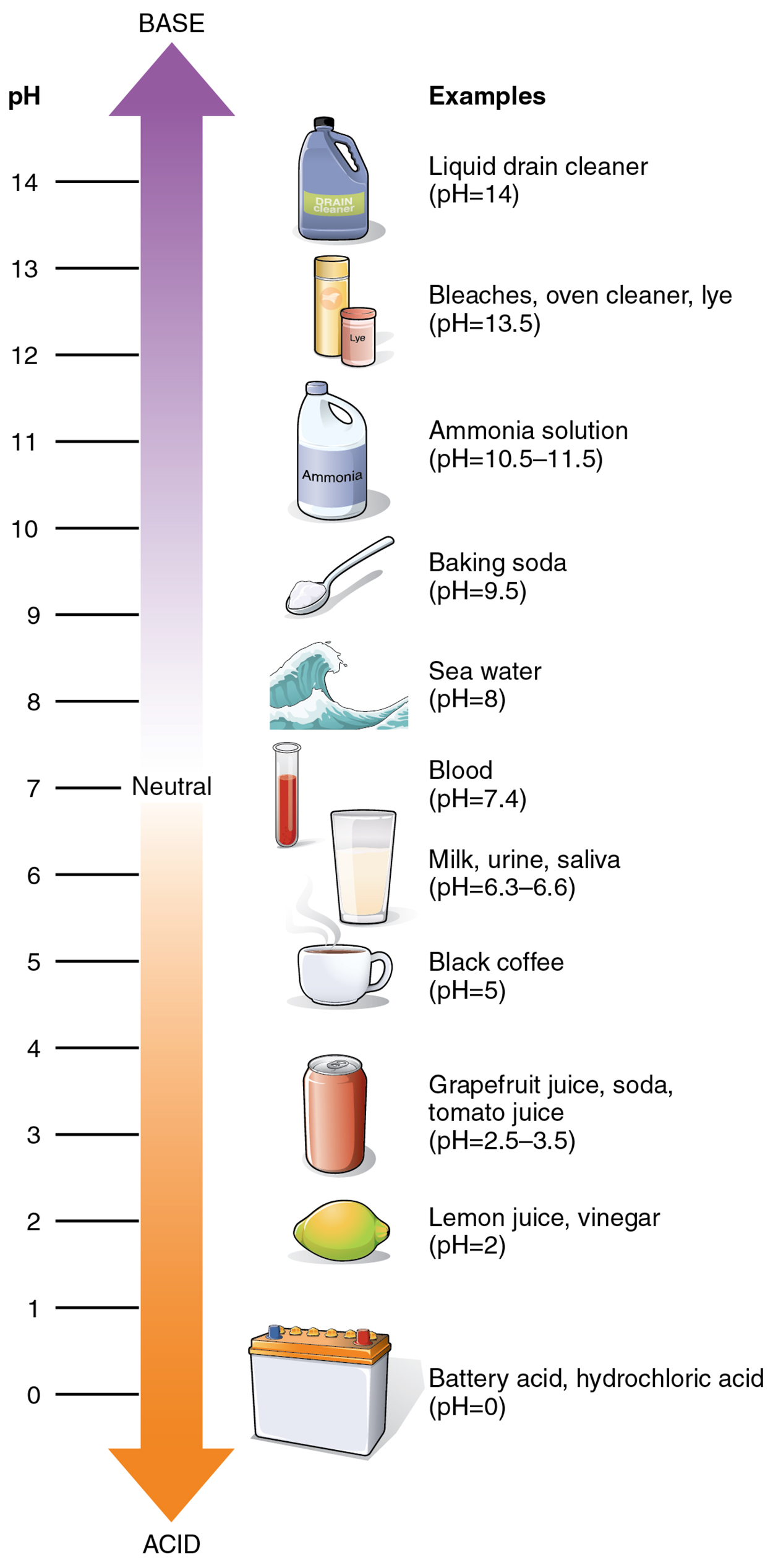
The pH of human blood normally ranges from 7.35 to 7.45. At this slightly basic pH, blood can reduce the acidity resulting from the carbon dioxide (CO₂) constantly being released into the bloodstream by the trillions of cells in the body. All cells of the body depend on homeostatic regulatory mechanisms to maintain this acid–base balance within its narrow range. Fluctuations, either too acidic or too alkaline, can lead to life-threatening disorders.
The human body has several homeostatic mechanisms for this regulation that you will learn about in future lessons, involving breathing, the excretion of chemicals in urine, and the internal release of chemicals collectively called buffers into body fluids. A buffer is a solution of a weak acid and its conjugate base. A buffer can neutralize small amounts of acids or bases, thereby maintaining the overall pH of the solution.
EXAMPLE
If there is even a slight decrease below 7.35 in the pH of a bodily fluid, the buffer will act as a weak base and bind the excess hydrogen ions. In contrast, if pH rises above 7.45, the buffer will act as a weak acid and contribute hydrogen ions.Fluctuations outside of this narrow blood pH range do occur, however. Excessive acidity of the blood and other body fluids is known as acidosis. The cause of acidosis is generally a buildup of CO₂ in the body due to excessive CO₂ production and/or limited removal or buffering. Excessive alkalinity of the blood and other body fluids is known as alkalosis. The general cause of alkalosis is a decrease of CO₂ in the blood due to decreased production and/or excessive removal. Both states can also be the result of factors such as underlying diseases or oral medications.
An organic compound is a substance that contains both carbon and hydrogen. Organic compounds are synthesized via covalent bonds within living organisms, including the human body. Carbon and hydrogen are the second and third most abundant elements in your body. You will soon discover how these two elements combine in the foods you eat, in the compounds that make up your body structure, and in the chemicals that fuel your functioning.
Organic molecules are important to the structure and function of the human body. Recall that organic molecules contain covalently bonded carbon and hydrogen atoms. These carbon skeletons (think of the “framework” of carbon) form four types of macromolecules that are important to human life:
| Functional group | Structural formula | Importance |
|---|---|---|
| Hydroxyl | —O—H | Hydroxyl groups are polar. They are components of all four types of organic compounds discussed in this unit. They are involved in dehydration synthesis reactions and hydrolysis reactions, which you will learn more about below. |
| Carboxyl |
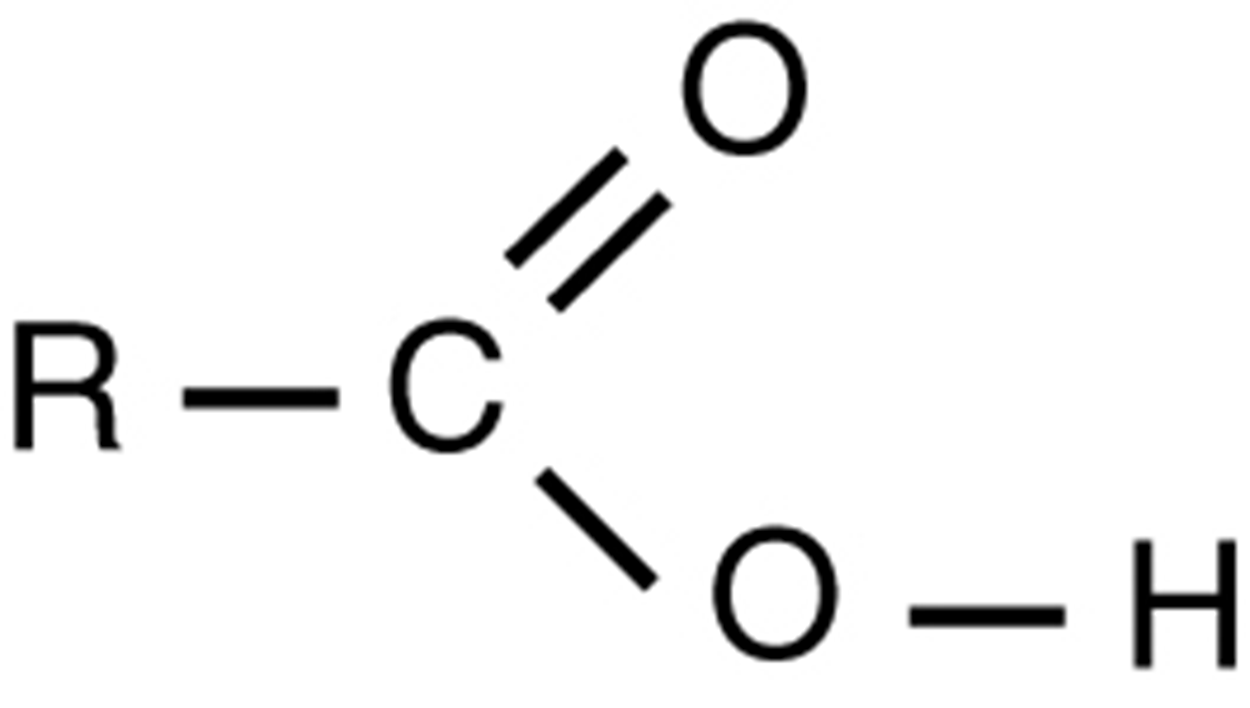
|
Carboxyl groups are found within fatty acids, amino acids, and many other acids. |
| Amino | —N—H₂ | Amino groups are found within amino acids, the building blocks of proteins. |
| Methyl | —C—H₃ | Methyl groups are found within amino acids. |
| Phosphate | —P—O₄²⁻ | Phosphate groups are found within phospholipids and nucleotides. |
Biological macromolecules are large molecules necessary for life that are built from smaller organic molecules. There are four major biological macromolecule classes (carbohydrates, lipids, proteins, and nucleic acids). Each is an important cell component and performs a wide array of functions. Combined, these molecules make up the majority of a cell’s dry mass (recall that water makes up the majority of its complete mass). Biological macromolecules are organic, meaning they contain carbon and are bound to hydrogen, and may contain oxygen, nitrogen, and additional minor elements.
Monomers (mono, one; mer, part), the basic units for building larger molecules, form polymers (poly, many), which are two or more chemically bonded monomers. Monomers and polymers are the foundation of biological macromolecules of the human body (including carbohydrates, lipids, proteins, and nucleic acids).
Carbon’s affinity for covalent bonding means that many distinct and relatively stable organic molecules readily form larger, more complex molecules. Any large molecule is referred to as a macromolecule (macro, large), and the organic compounds in this section all fit this description. However, some macromolecules are made up of several “copies” of monomers through dehydration synthesis, which is a reaction in which one reactant gives up an atom of hydrogen and another reactant gives up a hydroxyl group (OH) in the synthesis (creation) of a new product. Like beads in a long necklace, these monomers link by covalent bonds to form long polymers, and polymers can be broken apart by hydrolysis, which is a reaction in which a molecule of water disrupts a compound, breaking its bonds and decomposing the compound into two products. Almost every organic macromolecule forms monomers and polymers.
Dehydration and hydrolysis reactions are catalyzed, or “sped up,” by specific enzymes; dehydration reactions involve the formation of new bonds, requiring energy, whereas hydrolysis reactions break bonds and release energy. These reactions are similar for most macromolecules, but each monomer and polymer reaction is specific for its class of molecule.
EXAMPLE
Catalytic enzymes in the digestive system hydrolyze or break down the food we ingest into smaller molecules. This allows cells in our body to easily absorb nutrients in the intestine. A specific enzyme breaks down each macromolecule. For instance, amylase, sucrase, lactase, or maltase break down carbohydrates. Enzymes called proteases, such as pepsin and peptidase, and hydrochloric acid break down proteins. Lipases break down lipids. These broken-down macromolecules provide energy for cellular activities.In future lessons, you will learn more about the four types of macromolecules that are important to human life: carbohydrates, lipids, proteins, and nucleic acids.
SOURCE: THIS TUTORIAL HAS BEEN ADAPTED FROM (1) OPENSTAX “BIOLOGY 2E”. ACCESS FOR FREE AT OPENSTAX.ORG/BOOKS/BIOLOGY-2E/PAGES/1-INTRODUCTION (2) OPENSTAX “ANATOMY AND PHYSIOLOGY 2E”. ACCESS FOR FREE AT OPENSTAX.ORG/BOOKS/ANATOMY-AND-PHYSIOLOGY-2E/PAGES/1-INTRODUCTION. LICENSING (1 & 2): CREATIVE COMMONS ATTRIBUTION 4.0 INTERNATIONAL.