Table of Contents |
This lesson covers a range of in vitro (laboratory) tests that involve antigen–antibody reactions in varying ways. These tests can be very valuable in identifying the presence or quantity of an antigen or antibody. For example, it may be useful to know whether antiserum produced using a new approach contains a sufficiently high concentration of antibody for clinical use. Identifying antibodies in blood samples can also be used to determine whether someone has been exposed to a disease or was vaccinated against it. Detecting antigens can help in diagnosing diseases but can be used for many other purposes as well, which you will learn about in this lesson and in future lessons.
Precipitin reactions are in vitro assays (tests to detect the presence or quantity of something) that produce a visible antigen–antibody complex (called a precipitin). These reactions are generally accomplished by adding soluble antigens to a test tube containing a solution of antibodies.
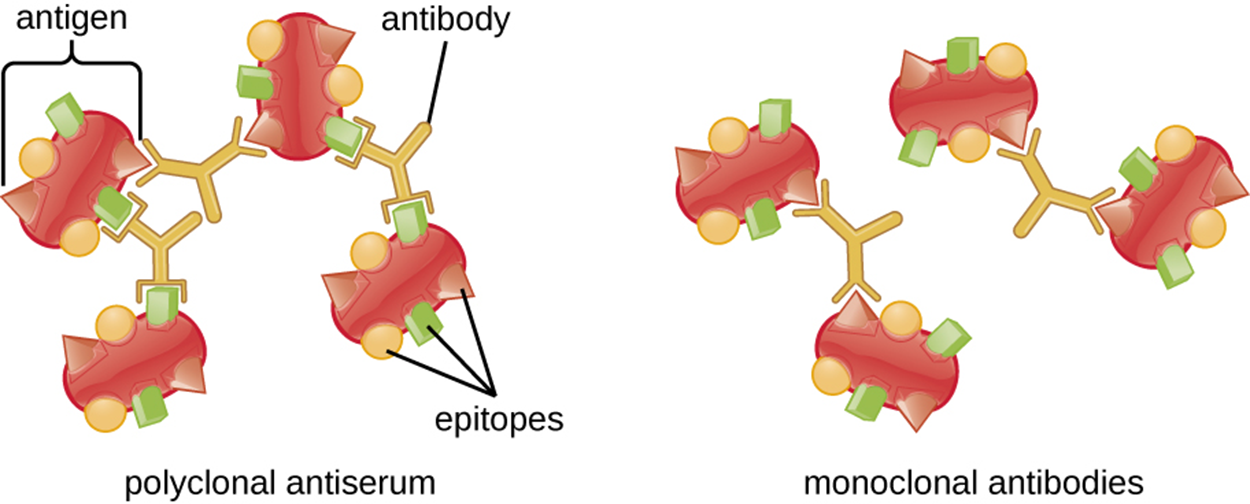
Each antibody has two arms that can bind to epitopes. When an antibody binds to epitopes on two separate antigens, then the separate antigens are bound together as they are each connected to part of the same antibody. As more and more antibodies bind, a lattice can form and this results in a precipitin.
The image below illustrates why polyclonal antibodies, rather than monoclonal antibodies, are generally used for these reactions. As the image shows, polyclonal antiserum contains antibodies that often bind to different epitopes and create stronger binding between antibodies than monoclonal antibodies that are selective for a particular epitope.
The amount of precipitation depends on a variety of factors. For example, antibodies with a high affinity for the antigen precipitate more than antibodies with weaker affinities.
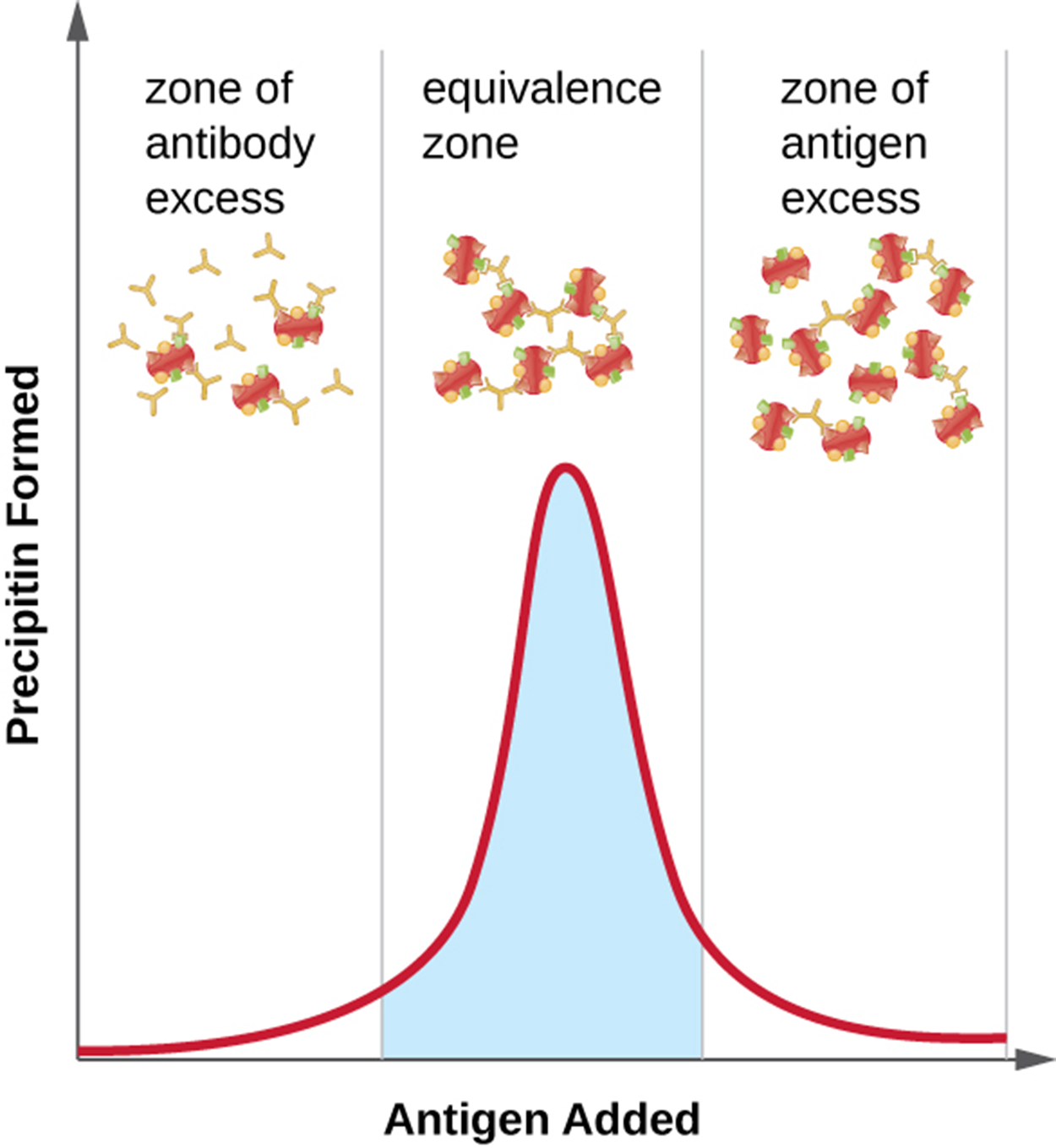
Additionally, the ratio of antibody to antigen is important and this is illustrated in the image below. The optimal ratio can vary considerably from one reaction to another and is affected by the number of epitopes on the antigen and the class of antibody.
To determine the optimal ratio, antigen is gradually added, and the amount of precipitin formed is measured quantitatively. Initially, there is an excess of antibody with relatively little antigen to bind (the zone of antibody excess). As more antigen is added, the reaction enters the equivalence zone (zone of equivalence) where optimal antigen–antibody interaction and maximal precipitation occur. If more antigen is added, the amount of antigen becomes excessive (the zone of antigen excess) and precipitation decreases.
The precipitin ring test is one approach used to quantify antigen concentration or the amount of antibody present in antiserum. The steps and image below explain how this test is performed.
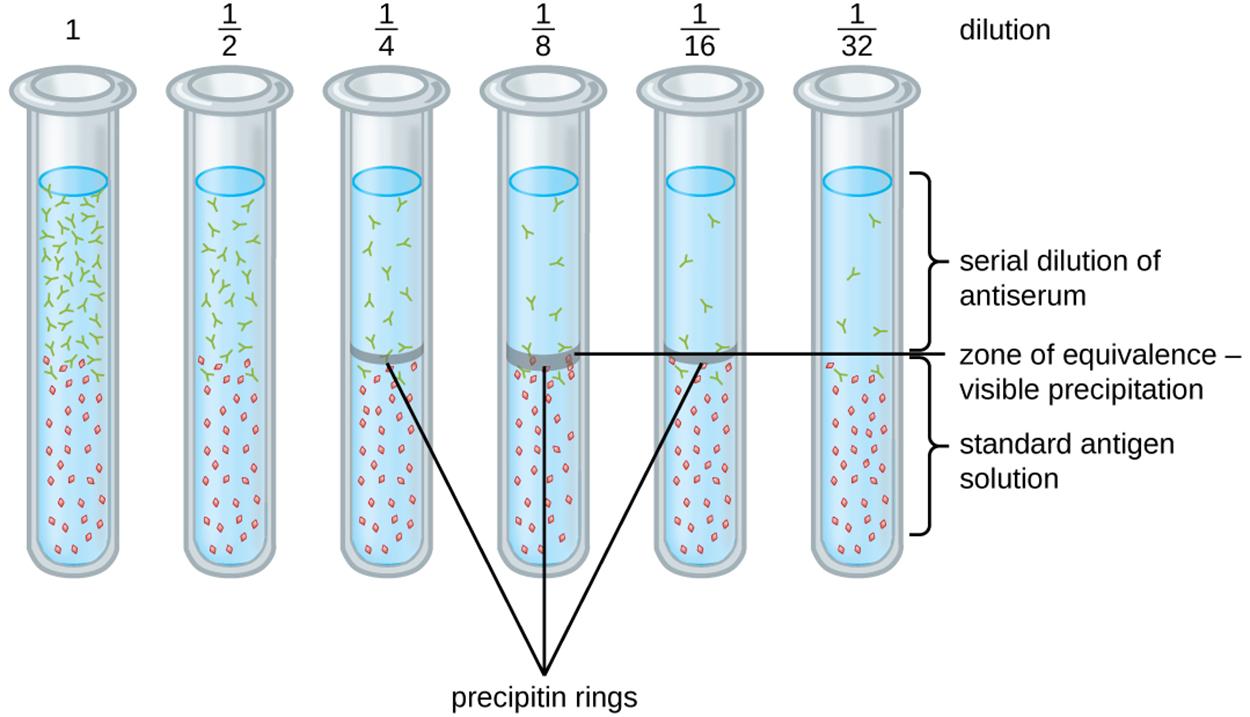
Note that the titer gives a measure of biological activity (e.g., how strongly a patient’s antibodies respond to an antigen) but does not give an absolute amount of antibody such as mass.
Drawbacks to the precipitin ring test include the following:
The Ouchterlony Assay (or double immunodiffusion assay) is similar to the precipitin ring test but performed using an agar gel matrix. This reduces the risk of mixing the antibody and antiserum solutions and reduces the amount of solution needed. Although more sensitive and quantitative methods are now available, this approach is a rapid, qualitative test that is especially useful in testing for cross-reactivity. The steps and image below explain the procedure.
To test for cross-reactivity, closely related antigens can be used with the same antiserum to determine which combinations form precipitin arcs.
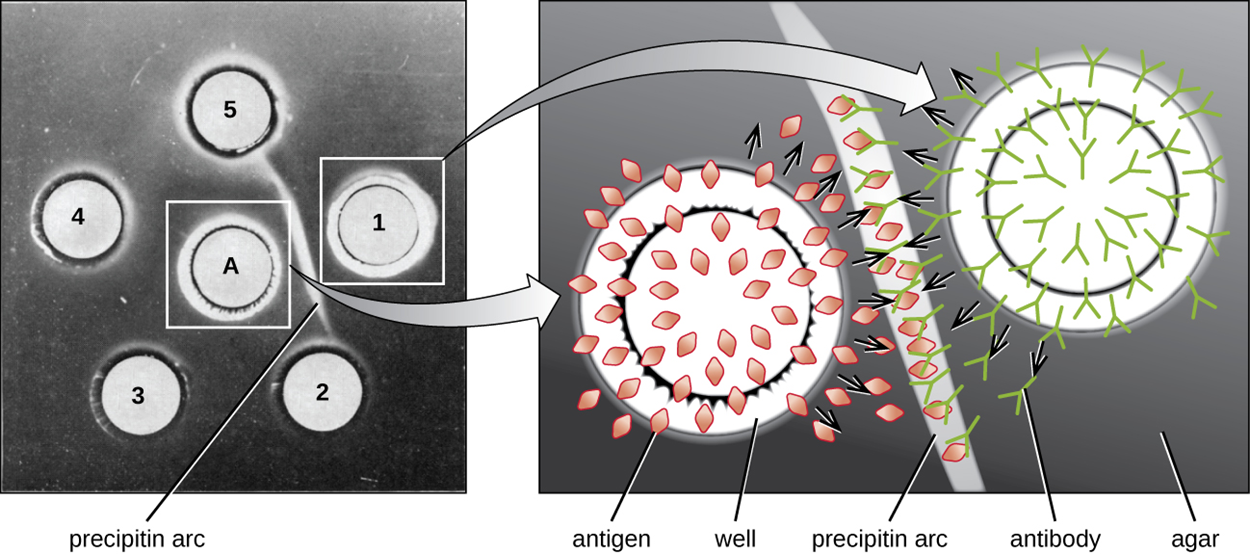
The image below shows antigen in well A on the left surrounded by wells of antiserum labeled 1 through 5. A precipitin arc is visible between wells A and 1, indicating that these react. On the right, a close-up shows how the antigen and antibody move from the wells into the agar gel and bind along the precipitin arc.
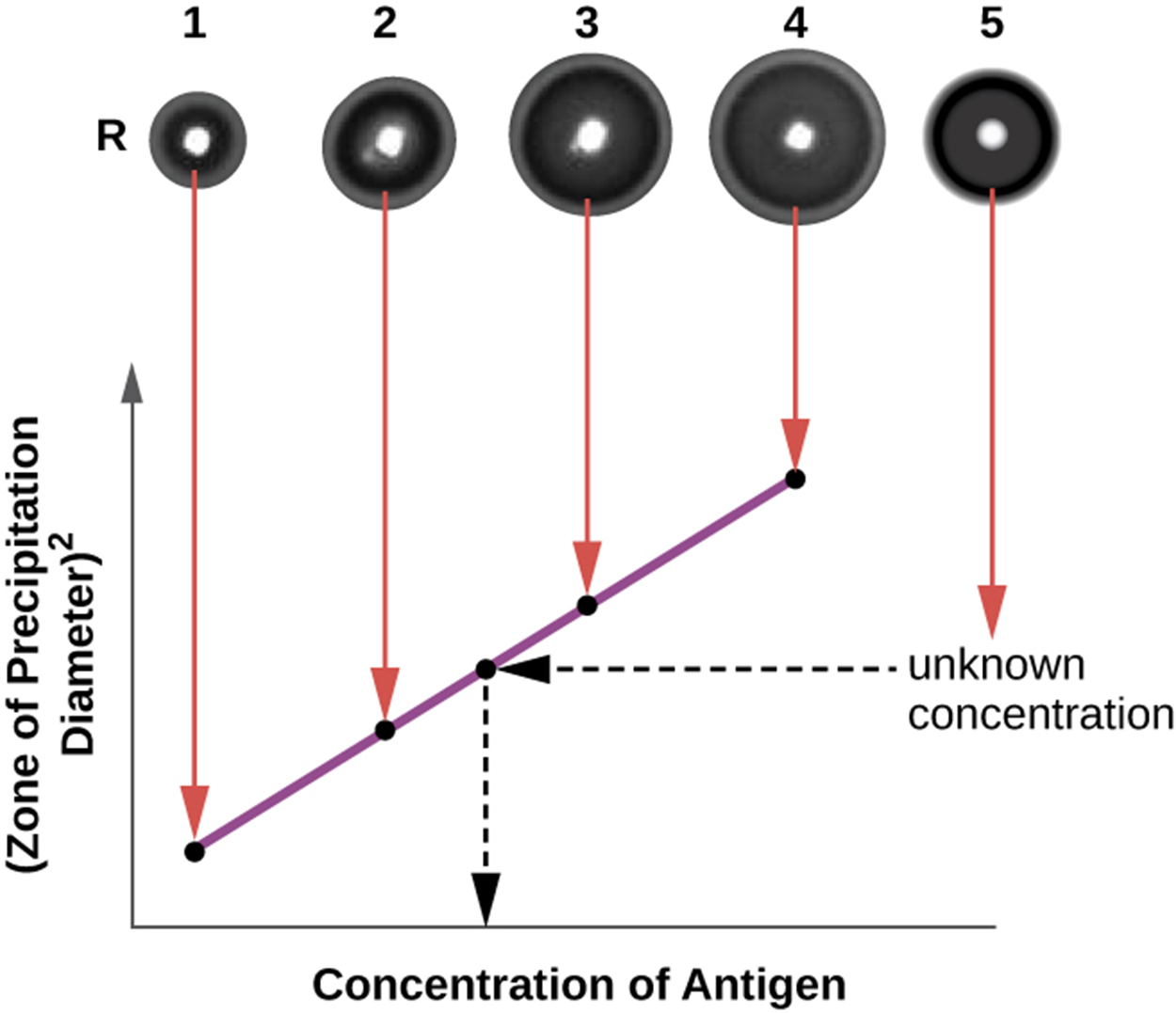
The radial immunodiffusion (RID) assay is similar to the Ouchterlony assay but is used to precisely quantify antigen concentration. The test can also be used to determine concentrations of serum proteins. The steps are summarized below and in the image.
The image shows four wells (numbered 1 through 4) containing antigen samples of known concentration. They are used to produce a standard curve relating the concentration of antigen to the zone of precipitation diameter squared. Well 5 contains an antigen sample of unknown concentration that can be compared to the standard curve to determine its antigen concentration.
A flocculation assay is similar to a precipitin reaction except that it involves insoluble antigens such as lipids that produce flocculants instead of precipitins. A flocculant is a lattice of antigen and antibody visible as flocculation (foaming) in the test tube fluid.
Neutralizing antibodies coat virions and prevent them from binding and infecting cells. In some cases, large antibody–virus complexes can form that can be removed by phagocytosis. Neutralization can be used in neutralization assays, which can be used to diagnose viral infections.
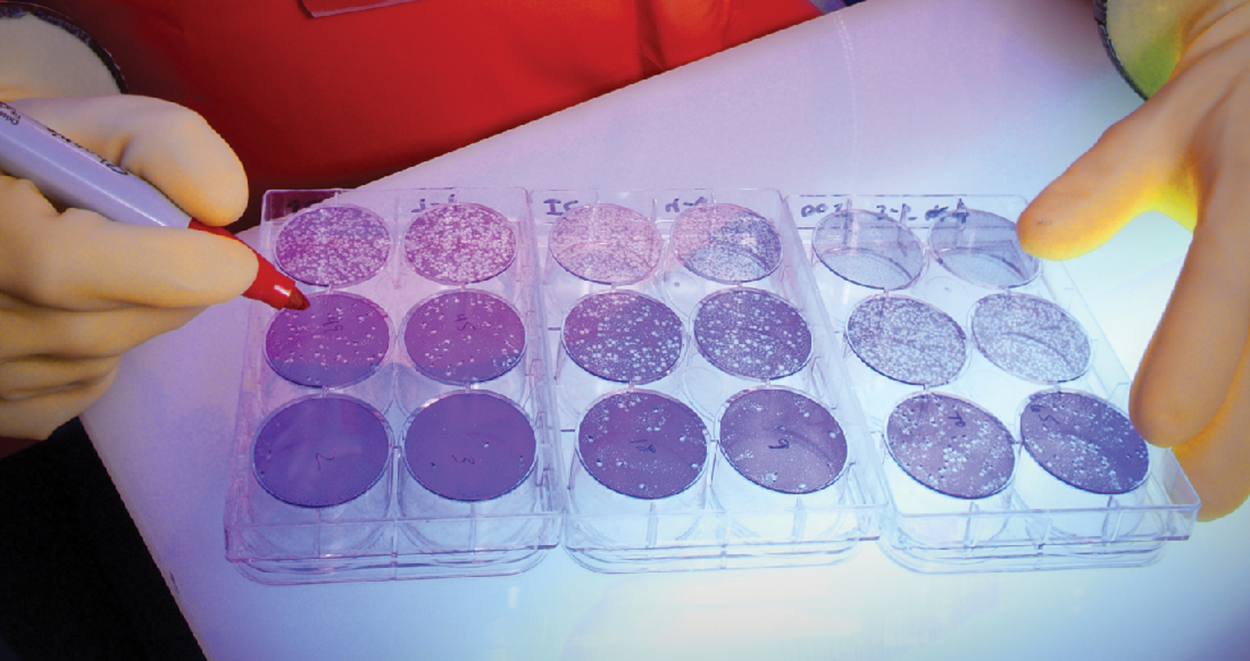
When viruses do infect cells, they often cause damage and sometimes cause lysis (bursting) or death. Changes in cells caused by viral infection (cytopathic effects) can be visualized by growing host cells in a petri dish, covering the cells with a thin layer of agar, and then adding viruses. As the viruses spread and kill cells, clear areas form.
The steps of a neutralization assay are summarized below.
The image below shows an example of a neutralization assay. The clear areas in the wells represent plaques where viruses have killed cells. Therefore, these are areas with higher numbers of active viruses than wells without clear patches. The wells contain patient serum and therefore the assay shows whether the patient has high concentrations of virus-neutralizing antibodies (and therefore a well with few or no clear areas) or low to nonexistent concentrations of virus-neutralizing antibodies (and therefore a well with large or entirely clear areas).
Immunoelectrophoresis (IEP) can be used to examine abnormal protein electrophoresis patterns. As discussed in earlier lessons, electrophoresis involves placing samples on a gel and applying an electrical current. Proteins travel through the gel at varying rates depending on their size and charge.
The first step in IEP is to use a polyacrylamide gel electrophoresis (PAGE) assay to separate proteins in a sample. Next, antisera against selected serum proteins can be added to troughs running parallel to the electrophoresis track. When antibodies and protein antigens interact, precipitin arcs form. This allows proteins of interest to be identified.
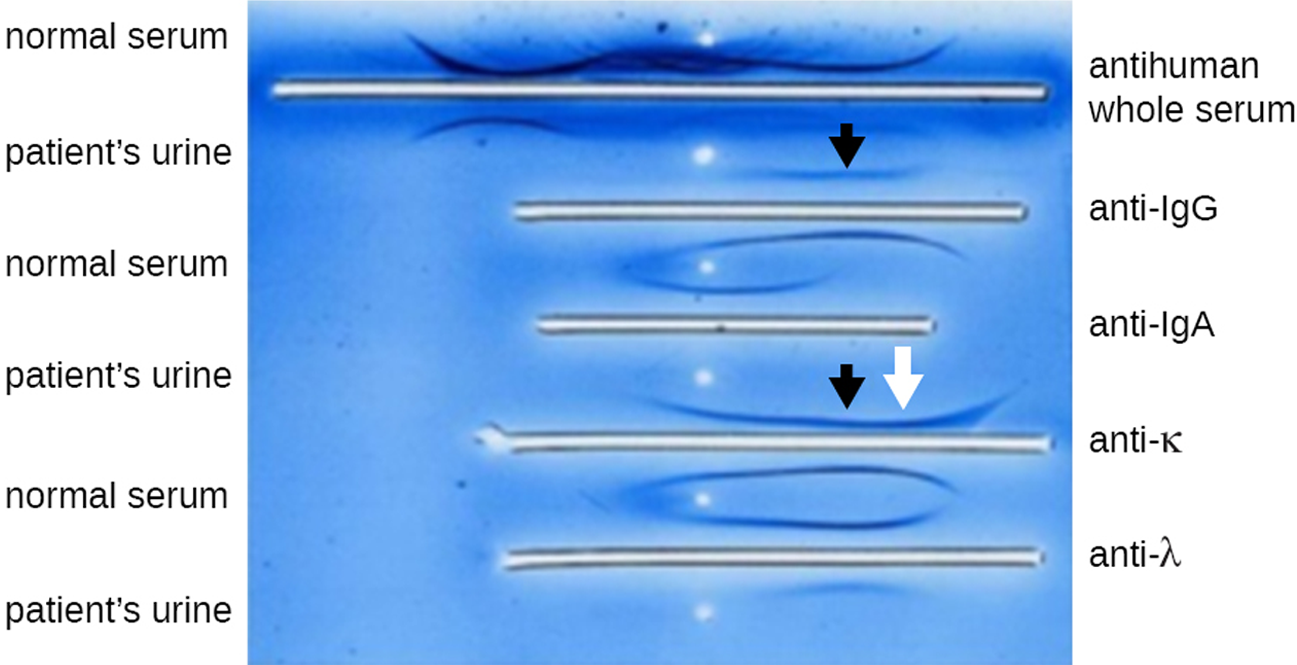
The photo below shows an IEP of urine. After electrophoresis, antisera were added to the troughs and precipitin arcs formed. The arrows indicate skewed arcs (those that are different from normal serum), which are helpful in diagnosing multiple myeloma. This is a cancer of antibody-secreting cells that causes the cells to produce abnormal antibodies called monoclonal proteins (M proteins) instead of healthy antibodies. These M proteins cause the distinctive skewed arcs shown in the photo.
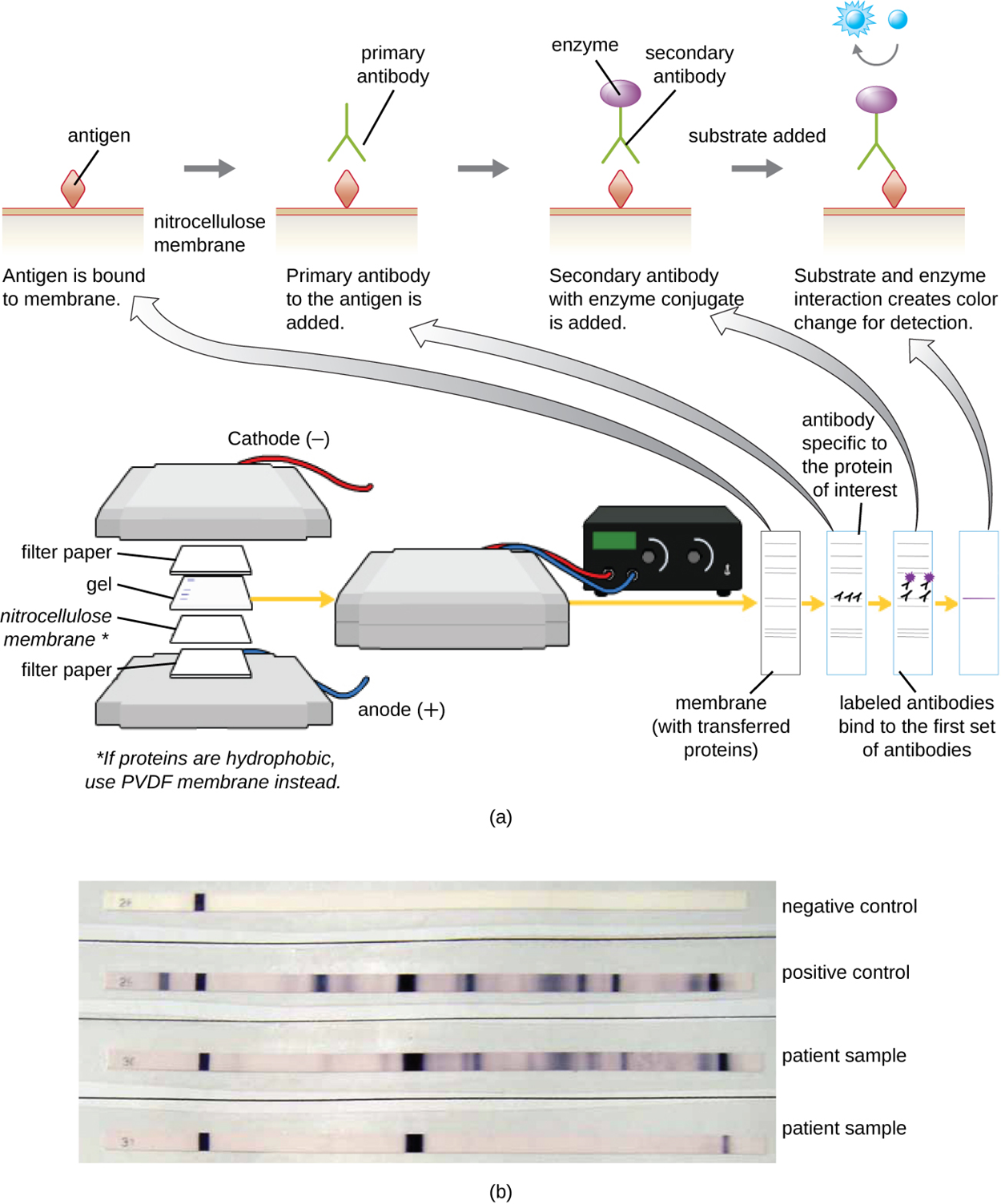
Protein gel electrophoresis can be followed by the addition of antibodies to identify specific proteins. This technique, described in the steps below, is called the western blot.
The image below shows the steps of a western blot in part (a) and an example of a western blot test for antibodies to HIV in part (b). The bands on the western blot indicate the presence of the protein of interest, which takes up stain.
Polyclonal antibodies are generally used for western blot assays because they are able to bind to multiple epitopes of the primary antigen and are therefore more sensitive than monoclonal antibodies. However, monoclonal antibodies can also be used when there is a need to target specific epitopes.
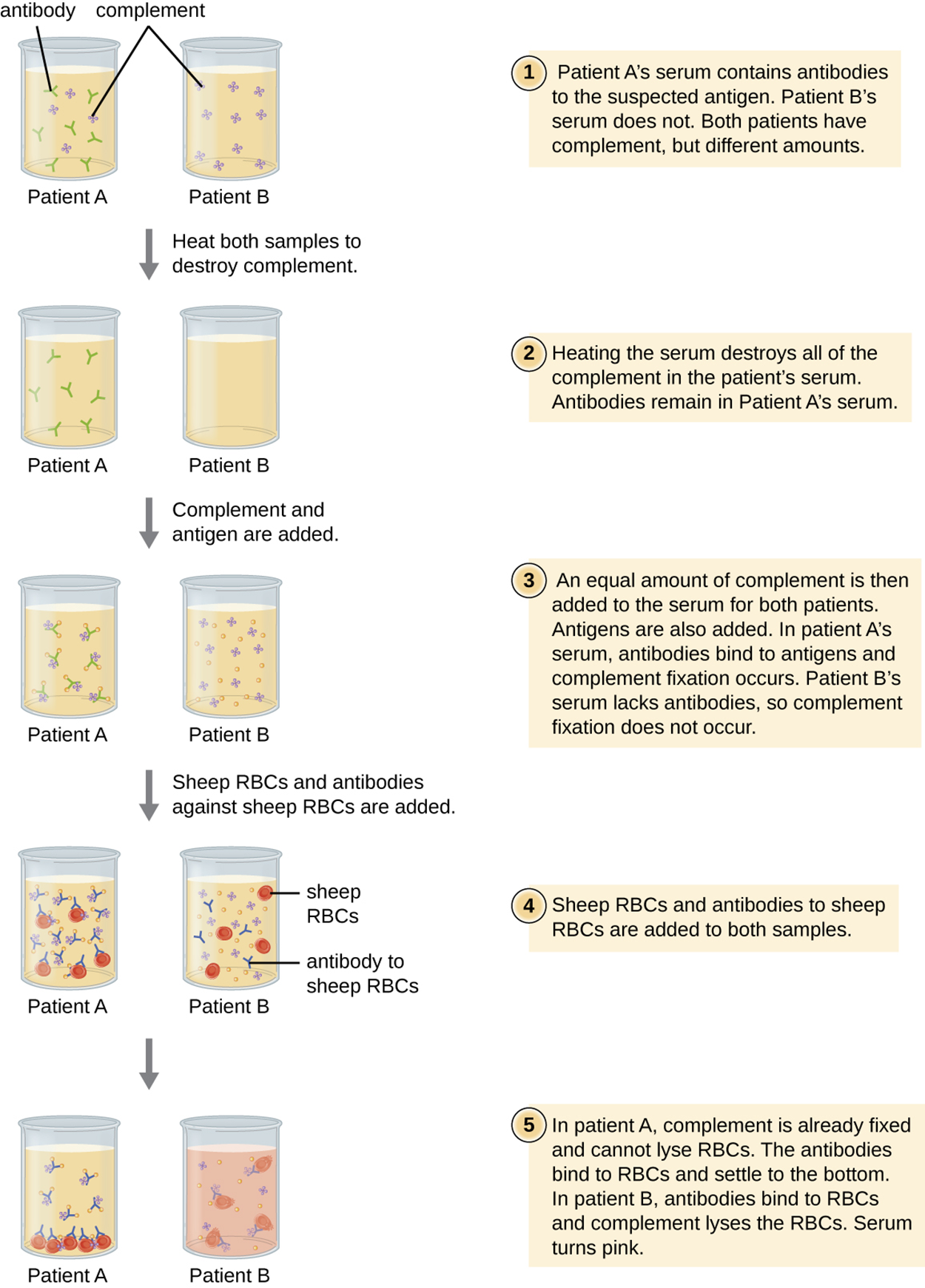
A complement fixation test allows the detection of antibodies against specific pathogens, including those that are difficult to culture in the lab. This test is possible because an important function of antibodies is to activate complement and initiate the complement cascade. Complement proteins play a variety of roles, including lytic activity that can be used to detect the presence of antibodies against specific antigens in serum.
In the complement fixation test, red blood cells are often used as indicator cells because they release the red pigment hemoglobin upon lysis, and even a small number of lysed cells release enough hemoglobin to produce a pink color.
The test is explained in the steps and image below. The steps summarize the major events, whereas the figure provides more detail about how the test is performed in a specific example.
The table below compares the mechanisms of assays discussed in this lesson and provides examples of each.
| Mechanisms of Select Antibody–Antigen Assays | ||
|---|---|---|
| Type of Assay | Mechanism | Examples |
| Precipitation | Antibody binds to soluble antigen, forming a visible precipitin | Precipitin ring test to visualize lattice formation in solution |
| Immunoelectrophoresis to examine distribution of antigens following electrophoresis | ||
| Ouchterlony assay to compare diverse antigens | ||
| Radial immunodiffusion assay to quantify antigens | ||
| Flocculation | Antibody binds to insoluble molecules in suspension, forming visible aggregates | VDRL (Venereal Disease Research Laboratory) test for syphilis |
| Neutralization | Antibody binds to virus, blocking viral entry into target cells and preventing formation of plaques | Plaque reduction assay for detecting the presence of neutralizing antibodies in patient sera |
| Complement activation | Antibody binds to antigen, inducing complement activation and leaving no complement to lyse red blood cells | Complement fixation test for patient antibodies against hard-to-culture bacteria such as Chlamydia |
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “MICROBIOLOGY.” ACCESS FOR FREE AT openstax.org/details/books/microbiology. LICENSE: CC ATTRIBUTION 4.0 INTERNATIONAL.