Table of Contents |
A functional group is a specific grouping of atoms that has a specific property or function. Functional groups are responsible for both the physical and chemical properties of organic chemistry. Functional groups also dictate all the reactions of organic chemistry.
There are many different functional groups found in organic chemistry. Two of the main classes of functional groups are the oxygen-containing and nitrogen-containing functional groups. The oxygen-containing functional groups include alcohols, ethers, and carbonyls (aldehydes, ketones, carboxylic acids, and esters). The nitrogen-containing functional groups include amines and amides.
Oxygen is the most common element on Earth and unsurprisingly oxygen-containing functional groups are the most common functional groups in organic chemistry. Alcohols and ethers are the oxygen-containing functional groups that consist of carbon-oxygen single bonds. Aldehydes, ketones, carboxylic acids, and esters are the oxygen-containing functional groups that consist of carbon-oxygen double bonds.
The incorporation of an oxygen atom into carbon- and hydrogen-containing molecules leads to new functional groups and new families of compounds. When the oxygen atom is attached by single bonds, the molecule is either alcohol or aether.
Alcohols are derivatives of hydrocarbons in which an –OH group has replaced a hydrogen atom. Although all alcohols have one or more hydroxyl (–OH) functional groups, they do not behave like bases such as NaOH and KOH. NaOH and KOH are ionic compounds that contain OH– ions. Alcohols are covalent molecules; the –OH group in an alcohol molecule is attached to a carbon atom by a covalent bond.
Ethanol, CH CH
CH OH, also called ethyl alcohol, is a particularly important alcohol for human use. Ethanol is the alcohol produced by some species of yeast that is found in wine, beer, and distilled drinks. It has long been prepared by humans harnessing the metabolic efforts of yeasts in the fermentation of various sugars:
OH, also called ethyl alcohol, is a particularly important alcohol for human use. Ethanol is the alcohol produced by some species of yeast that is found in wine, beer, and distilled drinks. It has long been prepared by humans harnessing the metabolic efforts of yeasts in the fermentation of various sugars:
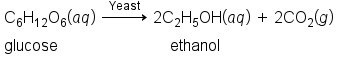
Alcohols containing two or more hydroxyl groups can also be made, as shown in the image below. Examples include 1,2-ethanediol (ethylene glycol, used in antifreeze) and 1,2,3-propanetriol (glycerine, used as a solvent for cosmetics and medicines).
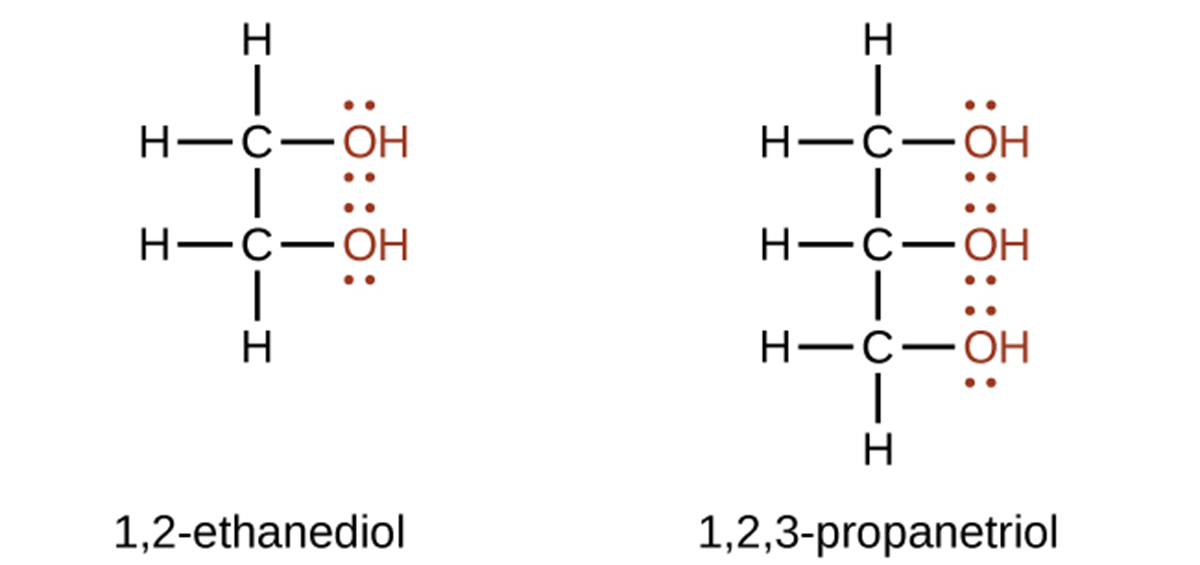
Ethers are compounds that contain the functional group –O–. The structure of ethylmethyl ether is shown below. The methyl group is highlighted in red (comes from the alkane name, methane).
In the general formula for ethers, R—O—R, the hydrocarbon groups (R) may be the same or different. Diethyl ether, the most widely used compound of this class, is a colorless, volatile liquid that is highly flammable. It was first used in 1846 as an anesthetic, but better anesthetics have now largely taken its place. Diethyl ether and other ethers are presently used primarily as solvents for gums, fats, waxes, and resins.
Tertiary-butyl methyl ether, C H
H OCH
OCH (abbreviated MTBE and italicized portions of names are not counted when ranking the groups alphabetically, so butyl comes before methyl in the common name), is used as an additive for gasoline. MTBE belongs to a group of chemicals known as oxygenates due to their capacity to increase the oxygen content of gasoline.
(abbreviated MTBE and italicized portions of names are not counted when ranking the groups alphabetically, so butyl comes before methyl in the common name), is used as an additive for gasoline. MTBE belongs to a group of chemicals known as oxygenates due to their capacity to increase the oxygen content of gasoline.
Carbonyls are the broad class of all carbon-oxygen double bonds (C=O) compounds. The trigonal planar carbon in the carbonyl group can attach to two other substituents leading to several subfamilies (aldehydes, ketones, carboxylic acids, and esters).
Carbonyl functional groups include aldehydes and ketones, which contain just the carbonyl (C=O) group. Carboxylic acids and esters consist of a carbonyl and a carbon-oxygen single bond. The general structure of a carbonyl is shown below:
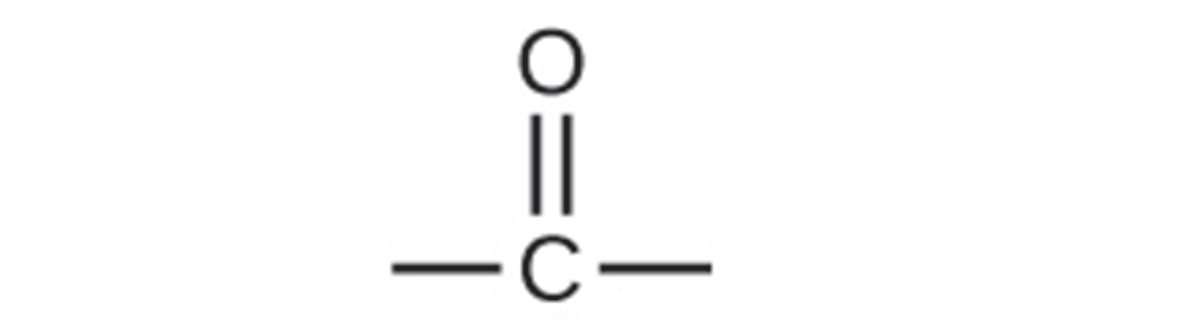
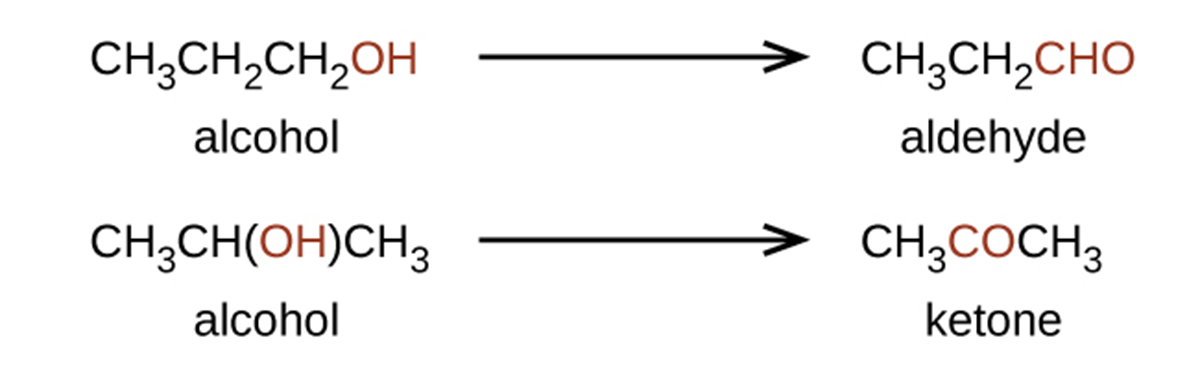
Both aldehydes and ketones contain a carbonyl group, a functional group with a carbon-oxygen double bond. In an aldehyde, the carbonyl group is bonded to at least one hydrogen atom. In a ketone, the carbonyl group is bonded to two carbon atoms:
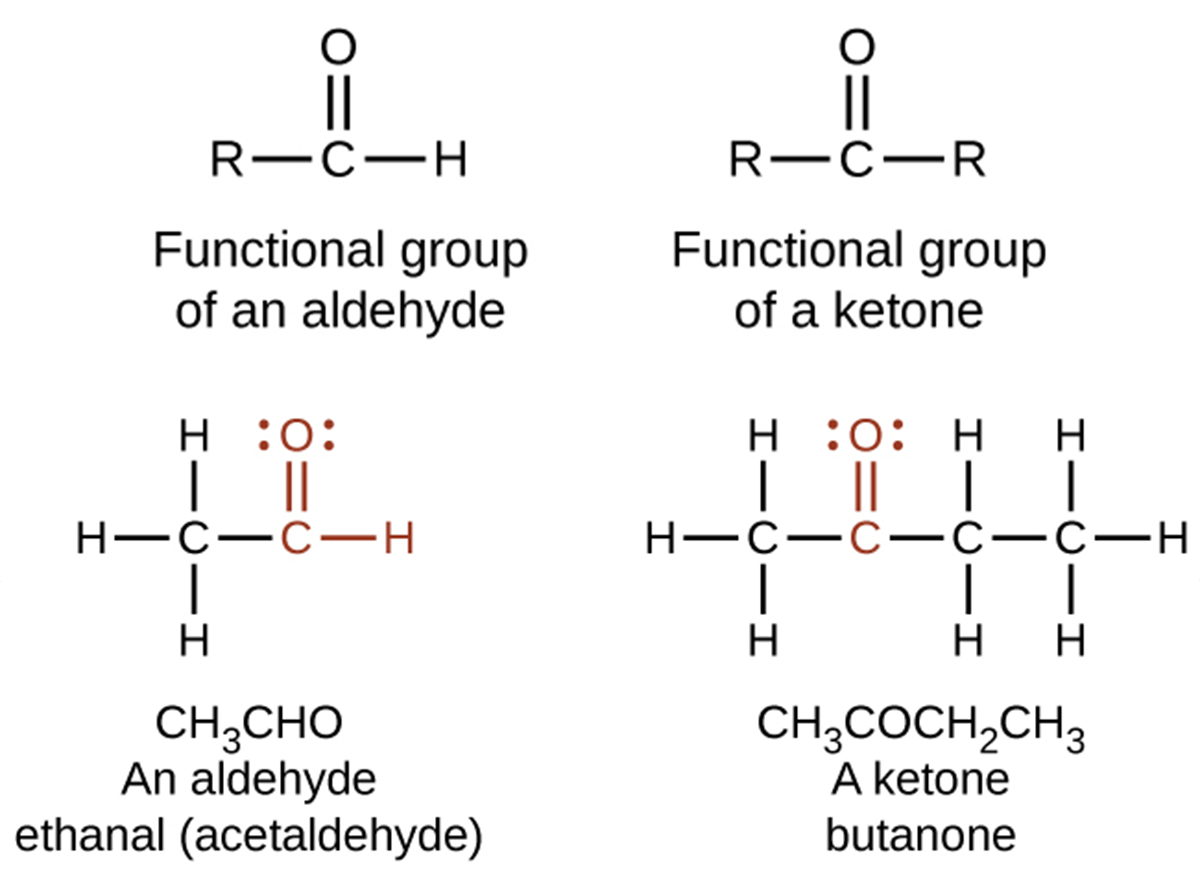
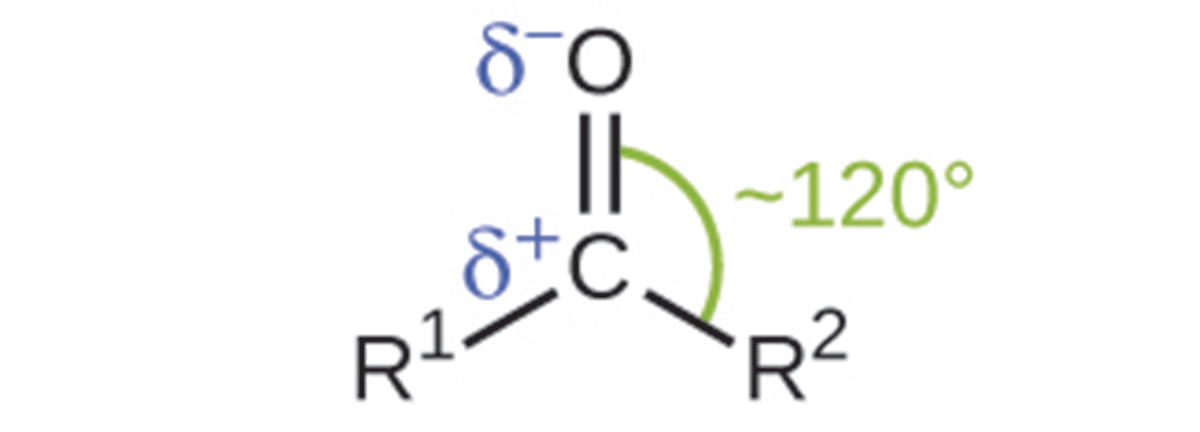
An aldehyde group can be represented by –CHO, and a ketone can be represented by –C(O)– or –CO–. In both aldehydes and ketones, the geometry around the carbon atom in the carbonyl group is trigonal planar, as shown in the above image.
Like the C=O bond in carbon dioxide, the C=O bond of a carbonyl group is polar (recall that oxygen is significantly more electronegative than carbon, and the shared electrons are pulled toward the oxygen atom and away from the carbon atom).
Many of the reactions of aldehydes and ketones start with the reaction between a Lewis base and the carbon atom at the positive end of the polar C=O bond to yield an unstable intermediate that subsequently undergoes one or more structural rearrangements to form the final product.
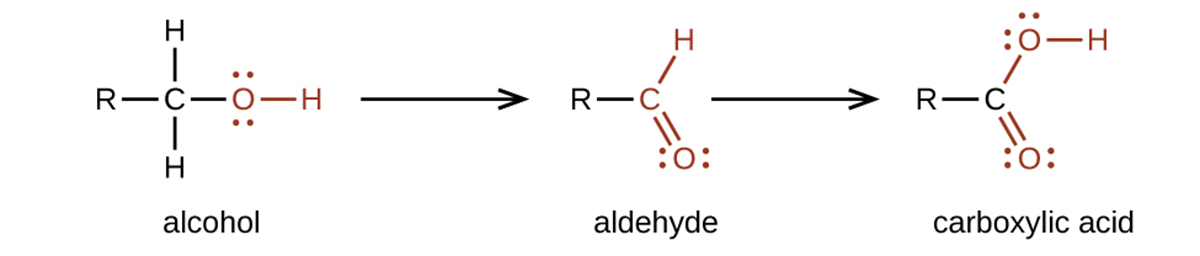
The importance of molecular structure in the reactivity of organic compounds is illustrated by the reactions that produce aldehydes and ketones. We can prepare a carbonyl group by oxidation of an alcohol, for organic molecules, oxidation of a carbon atom is said to occur when a carbon-hydrogen bond is replaced by a carbon-oxygen bond. The reverse reaction, replacing a carbon-oxygen bond with a carbon-hydrogen bond, is a reduction of that carbon atom.
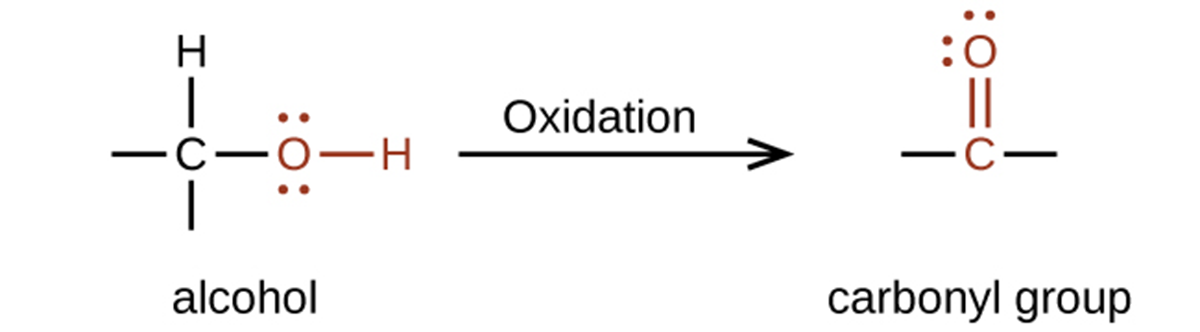
An alcohol with its –OH group bonded to a carbon atom that is bonded to none or one other carbon atom will form an aldehyde. An alcohol with its –OH group attached to two other carbon atoms will form a ketone. If three carbons are attached to the carbon bonded to the –OH, the molecule will not have a C–H bond to be replaced, so it will not be susceptible to oxidation.
Formaldehyde, an aldehyde with the formula HCHO, is a colorless gas with a pungent and irritating odor. It is sold in an aqueous solution called formalin, which contains about 37% formaldehyde by weight. Formaldehyde causes coagulation of proteins, so it kills bacteria (and any other living organism) and stops many of the biological processes that cause tissue to decay. Thus, formaldehyde is used for preserving tissue specimens and embalming bodies. It is also used to sterilize soil or other materials.
Dimethyl ketone, CH COCH
COCH , commonly called acetone, is the simplest ketone. It is made commercially by fermenting corn or molasses, or by oxidation of 2-propanol. Acetone is a colorless liquid. Among its many uses are as a solvent for lacquer (including fingernail polish), cellulose acetate, cellulose nitrate, acetylene, plastics, and varnishes; as a paint and varnish remover; and as a solvent in the manufacture of pharmaceuticals and chemicals.
, commonly called acetone, is the simplest ketone. It is made commercially by fermenting corn or molasses, or by oxidation of 2-propanol. Acetone is a colorless liquid. Among its many uses are as a solvent for lacquer (including fingernail polish), cellulose acetate, cellulose nitrate, acetylene, plastics, and varnishes; as a paint and varnish remover; and as a solvent in the manufacture of pharmaceuticals and chemicals.
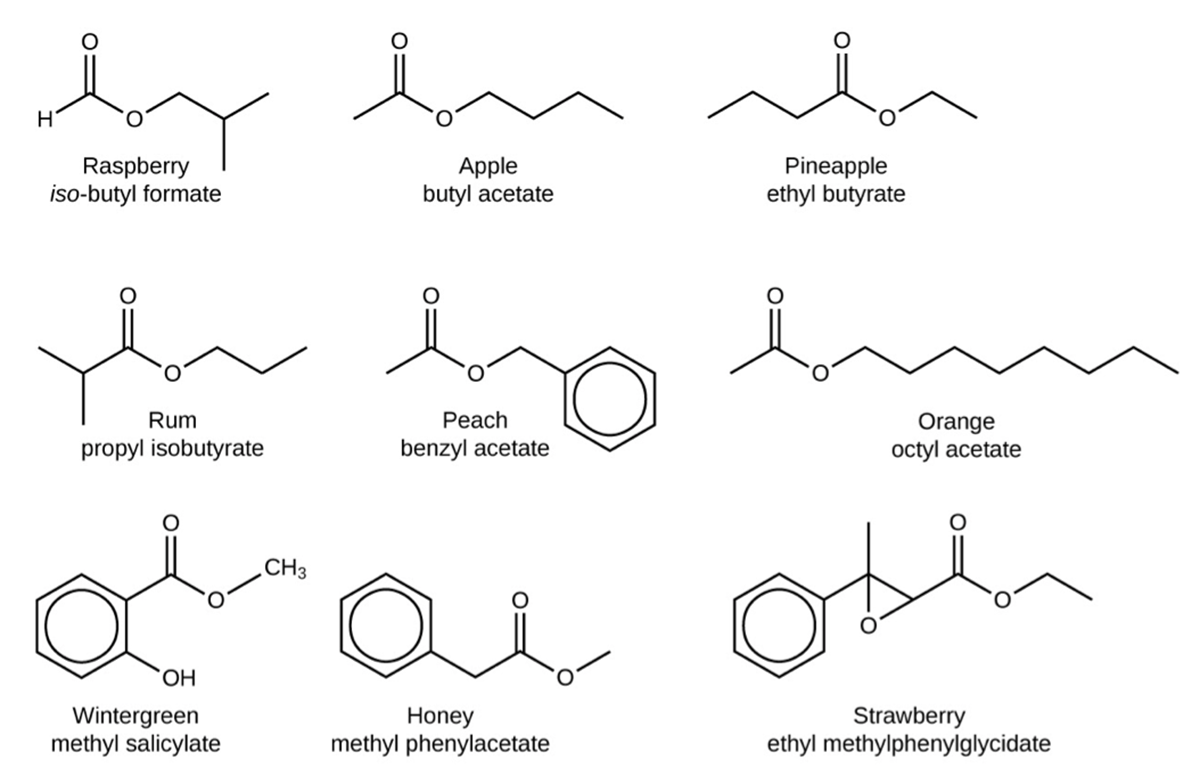
The odor of vinegar is caused by the presence of acetic acid, a carboxylic acid, in the vinegar. The odor of ripe bananas and many other fruits is due to the presence of esters, compounds that can be prepared by the reaction of a carboxylic acid with an alcohol.
Because esters do not have hydrogen bonds between molecules, they have lower vapor pressures than the alcohols and carboxylic acids from which they are derived and thus lower boiling points. Esters are usually volatile, meaning they are easily vaporized at room temperature. The aroma of the ester compounds shown below is due to their volatility.
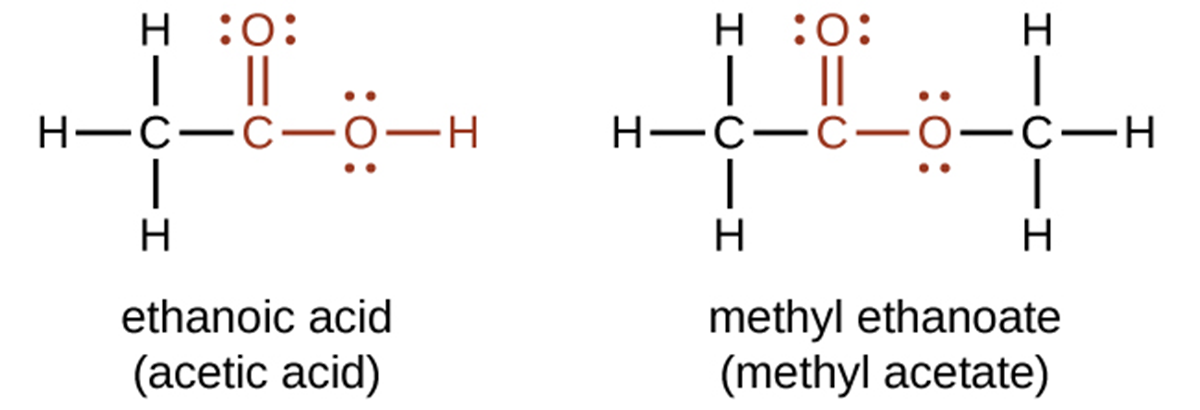
Both carboxylic acids and esters contain a carbonyl group with a second oxygen atom bonded to the carbon atom in the carbonyl group by a single bond. In a carboxylic acid, the second oxygen atom also bonds to a hydrogen atom (-COOH). In an ester, the second oxygen atom bonds to another carbon atom (-COO-). The functional groups for an acid and for an ester are shown in red in these formulas:
We prepare carboxylic acids by the oxidation of aldehydes or alcohols whose –OH functional group is located on the carbon atom at the end of the chain of carbon atoms in the alcohol:
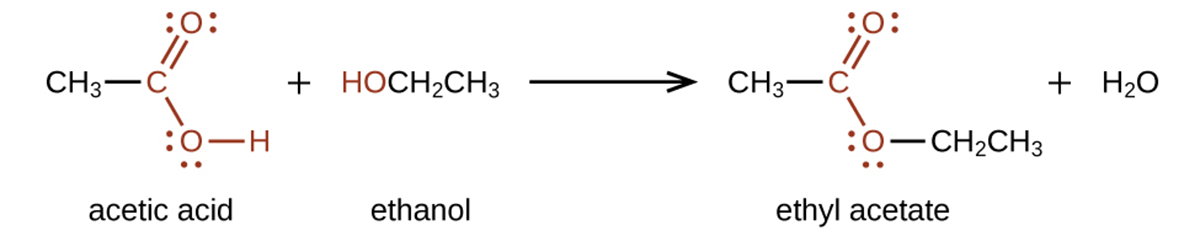
Esters are produced by the reaction of acids with alcohols. For example, the ester ethyl acetate, CH CO
CO CH
CH CH
CH , is formed when acetic acid reacts with ethanol:
, is formed when acetic acid reacts with ethanol:
EXAMPLE
The simplest carboxylic acid is formic acid, HCO H, known since 1670. Its name comes from the Latin word formicus, which means “ant”; it was first isolated by the distillation of red ants. It is partially responsible for the pain and irritation of ant and wasp stings and is responsible for a characteristic odor of ants that can be sometimes detected in their nests.
H, known since 1670. Its name comes from the Latin word formicus, which means “ant”; it was first isolated by the distillation of red ants. It is partially responsible for the pain and irritation of ant and wasp stings and is responsible for a characteristic odor of ants that can be sometimes detected in their nests.
Acetic acid, CH CO
CO H, constitutes 3–6% vinegar. Cider vinegar is produced by allowing apple juice to ferment without oxygen present. Yeast cells present in the juice carry out the fermentation reactions. The fermentation reactions change the sugar present in the juice to ethanol, then to acetic acid. Pure acetic acid has a penetrating odor and produces painful burns. It is an excellent solvent for many organic and some inorganic compounds, and it is essential in the production of cellulose acetate, a component of many synthetic fibers such as rayon.
H, constitutes 3–6% vinegar. Cider vinegar is produced by allowing apple juice to ferment without oxygen present. Yeast cells present in the juice carry out the fermentation reactions. The fermentation reactions change the sugar present in the juice to ethanol, then to acetic acid. Pure acetic acid has a penetrating odor and produces painful burns. It is an excellent solvent for many organic and some inorganic compounds, and it is essential in the production of cellulose acetate, a component of many synthetic fibers such as rayon.
EXAMPLE
The distinctive and attractive odors and flavors of many flowers, perfumes, and ripe fruits are due to the presence of one or more esters. Among the most important of the natural esters are fats (such as lard, tallow, and butter) and oils (such as linseed, cottonseed, and olive oils).Nitrogen is another common element on Earth and nitrogen-containing functional groups are the second most common functional groups in organic chemistry. Amines and amides are the most common nitrogen-containing functional groups.
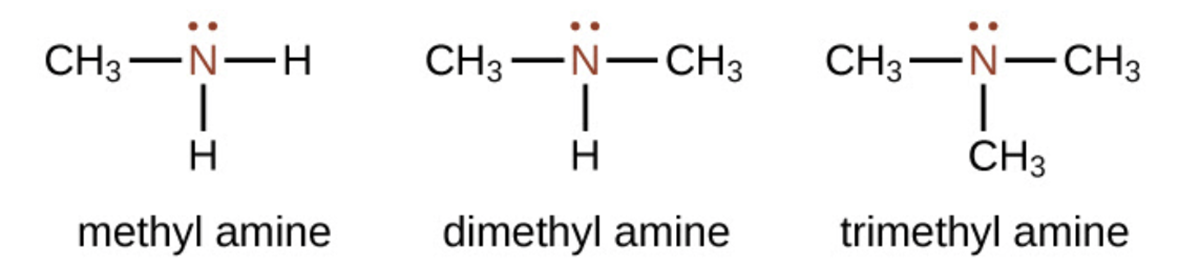
Amines are molecules that contain carbon-nitrogen bonds. The nitrogen atom in an amine has a lone pair of electrons and three bonds to other atoms, either carbon or hydrogen. Three common amines, methyl amine, dimethylamine, and trimethyl amine, are shown below.
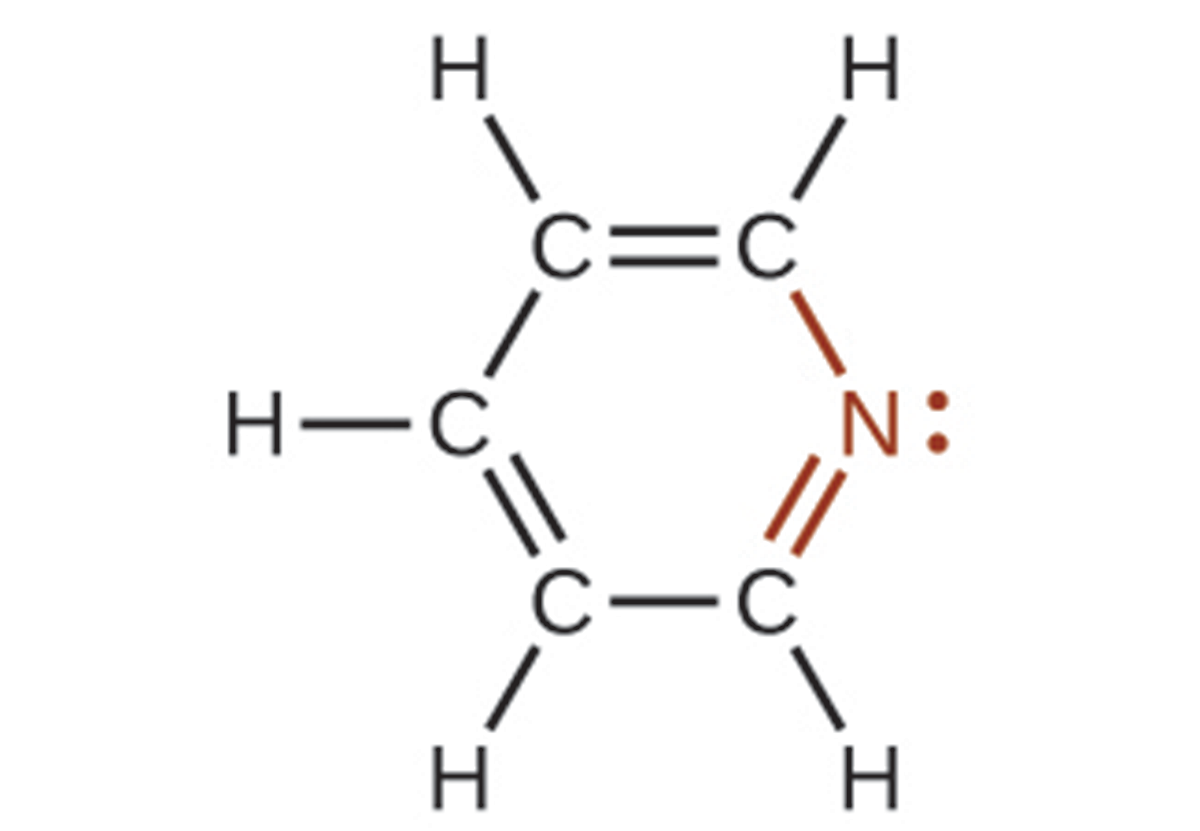
In some amines, the nitrogen atom replaces a carbon atom in an aromatic hydrocarbon. Pyridine is one such heterocyclic amine. A heterocyclic compound contains atoms of two or more different elements in its ring structure.
Like ammonia, amines are weak bases due to the lone pair of electrons on their nitrogen atoms as shown below:
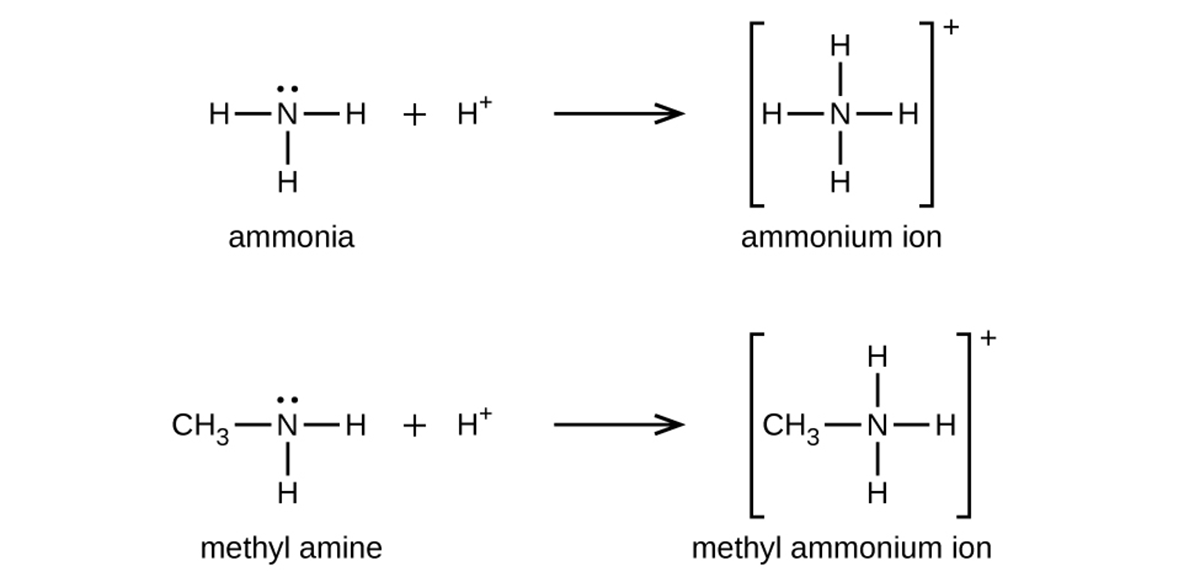
The basicity of an amine’s nitrogen atom plays an important role in much of the compound’s chemistry. Amine functional groups are found in a wide variety of compounds, including natural and synthetic dyes, polymers, vitamins, and medications, such as penicillin and codeine. They are also found in many molecules essential to life, such as amino acids, hormones, neurotransmitters, and DNA.
Amides are molecules that contain nitrogen atoms connected to the carbon atom of a carbonyl group.
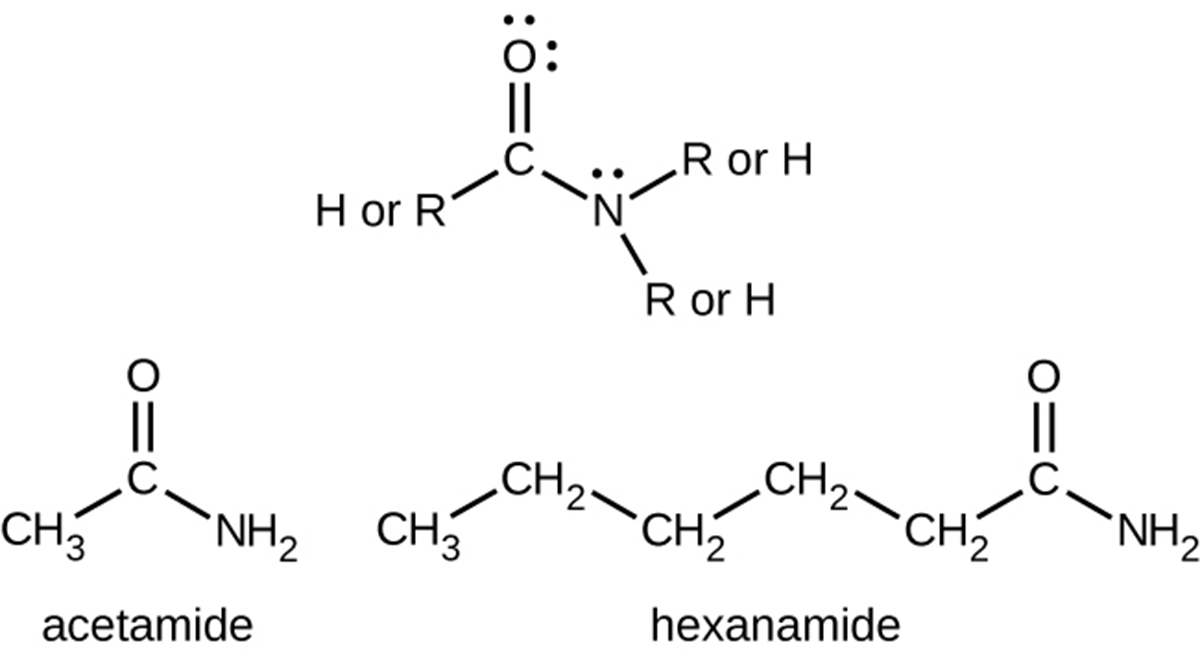
Amides can be produced when carboxylic acids react with amines or ammonia in a process called amidation. A water molecule is eliminated from the reaction, and the amide is formed from the remaining pieces of the carboxylic acid and the amine (note the similarity to the formation of an ester from a carboxylic acid and an alcohol discussed previously).
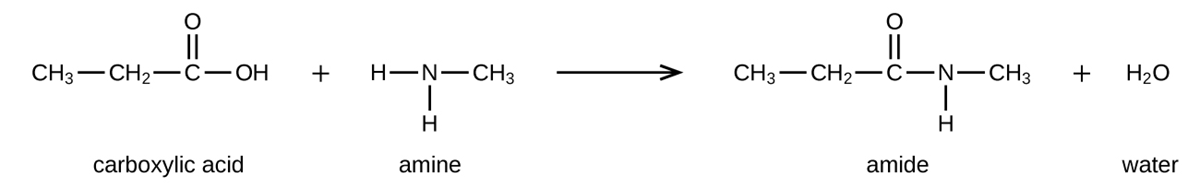
The reaction between amines and carboxylic acids to form amides is biologically important. It is through this reaction that amino acids (molecules containing both amine and carboxylic acid substituents) link together in a polymer to form proteins.
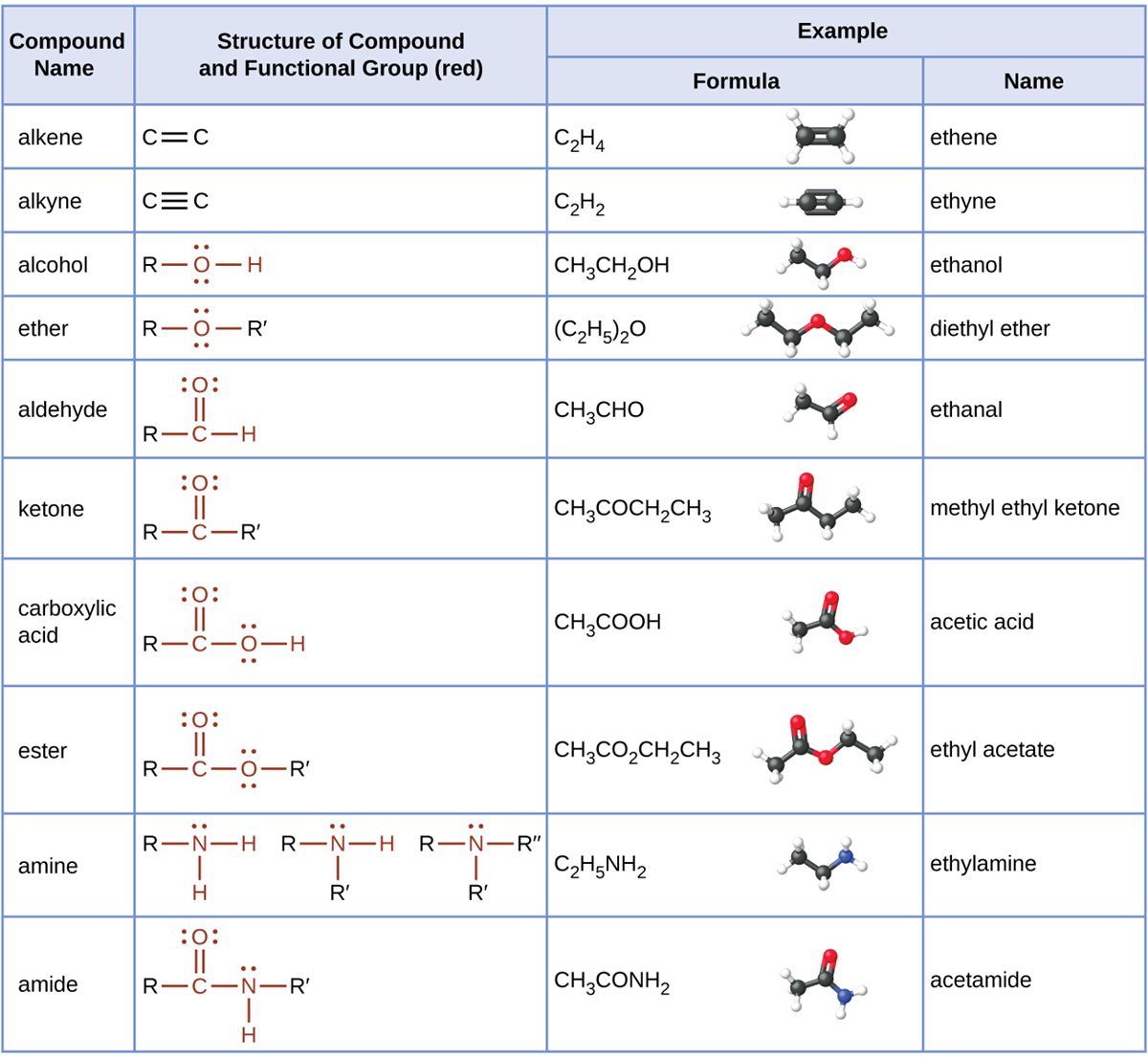
The table here summarizes the structures discussed in this and the previous lesson:
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “CHEMISTRY: ATOMS FIRST 2E”. ACCESS FOR FREE AT Chemistry: Atoms First 2e. LICENSE: CREATIVE COMMONS ATTRIBUTION 4.0 INTERNATIONAL