Table of Contents |
Fluorescent antibody (FA) techniques use a fluorogen (a fluorescent marker) attached to the constant region of an antibody so that the antibody functions as a reporter molecule that rapidly makes visible the location of antigens to which it binds. These techniques are quick, easy to see, and allow for binding with high specificity. They can even be used to label individual cells to quantify or isolate subsets of cells (sometimes using automated systems).
These techniques can be direct or indirect.
Direct fluorescent antibody (DFA) tests use a fluorescently labeled mAb to bind to and illuminate a target antigen, such as a bacterial structure. They can be used with patient samples such as throat swabs and can be performed on a microscope swab. This makes them quick and easy diagnostic approaches that work even with pathogens that cannot easily be cultured. They are especially useful in detecting bacteria.
The image below shows an example of a bacterial smear from a patient with a respiratory infection. Fluorescein (a green fluorescent dye) has been added to mAbs that have bound to the bacteria, making them clearly visible.
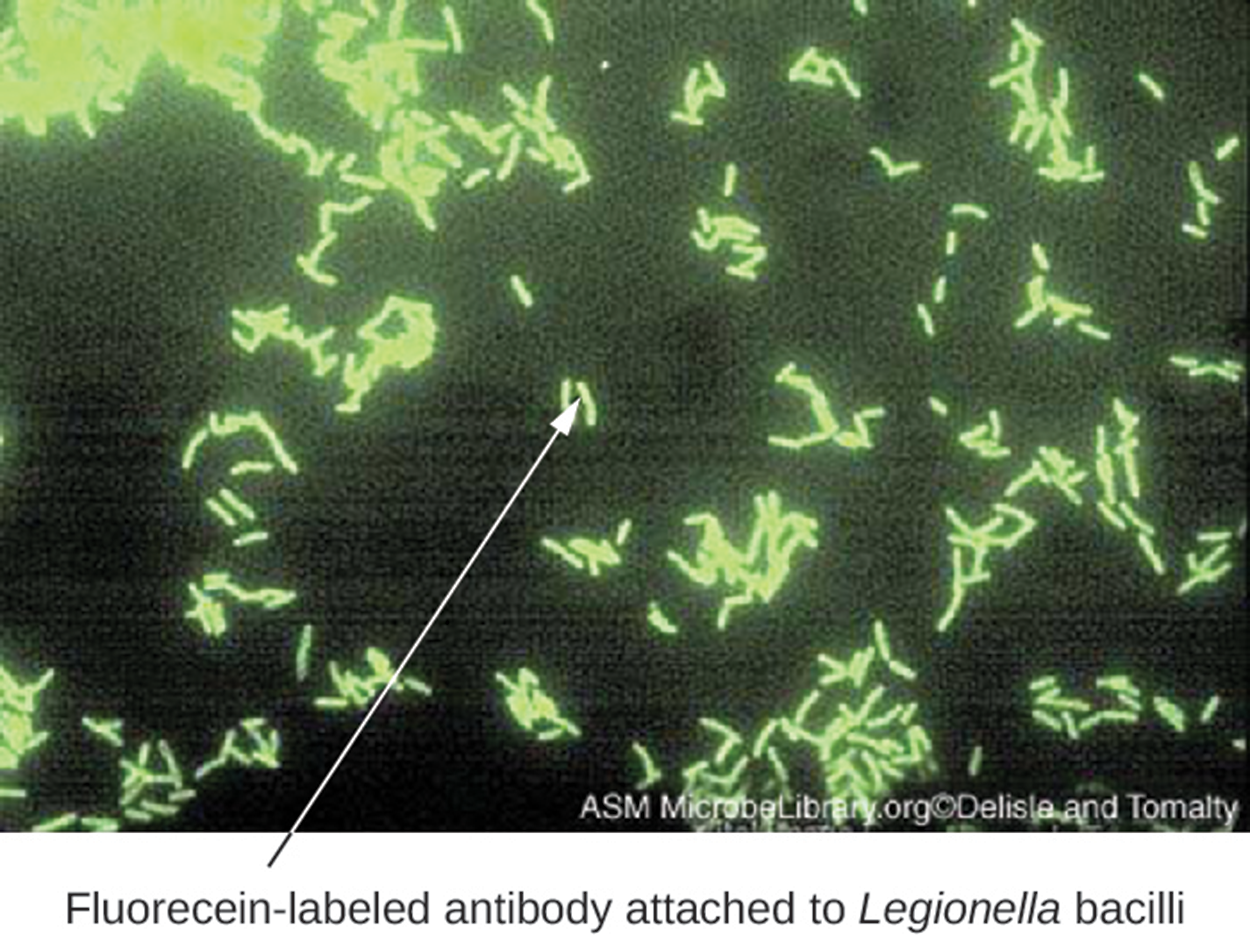
Indirect fluorescent antibody (IFA) tests are used to look for antibodies in patient serum, unlike DFA tests that detect antigens. A smear containing the antigen of interest (often a pathogen) is prepared on a glass slide. Patient serum is spread over the smear. If the serum contains antibodies, they bind to the antigens on the slide. The serum is washed off, and a secondary antibody is added. The secondary antibody is an antihuman immunoglobulin conjugated to a fluorogen. This antibody will bind to the primary antibodies, if present, indicating that the antigen was present in the serum.
The image below shows the steps involved in the IFA. Part (a) of the image shows the general steps for IFA. Part (b) shows a micrograph in which measles-infected cells are fluorescent green following an IFA test.
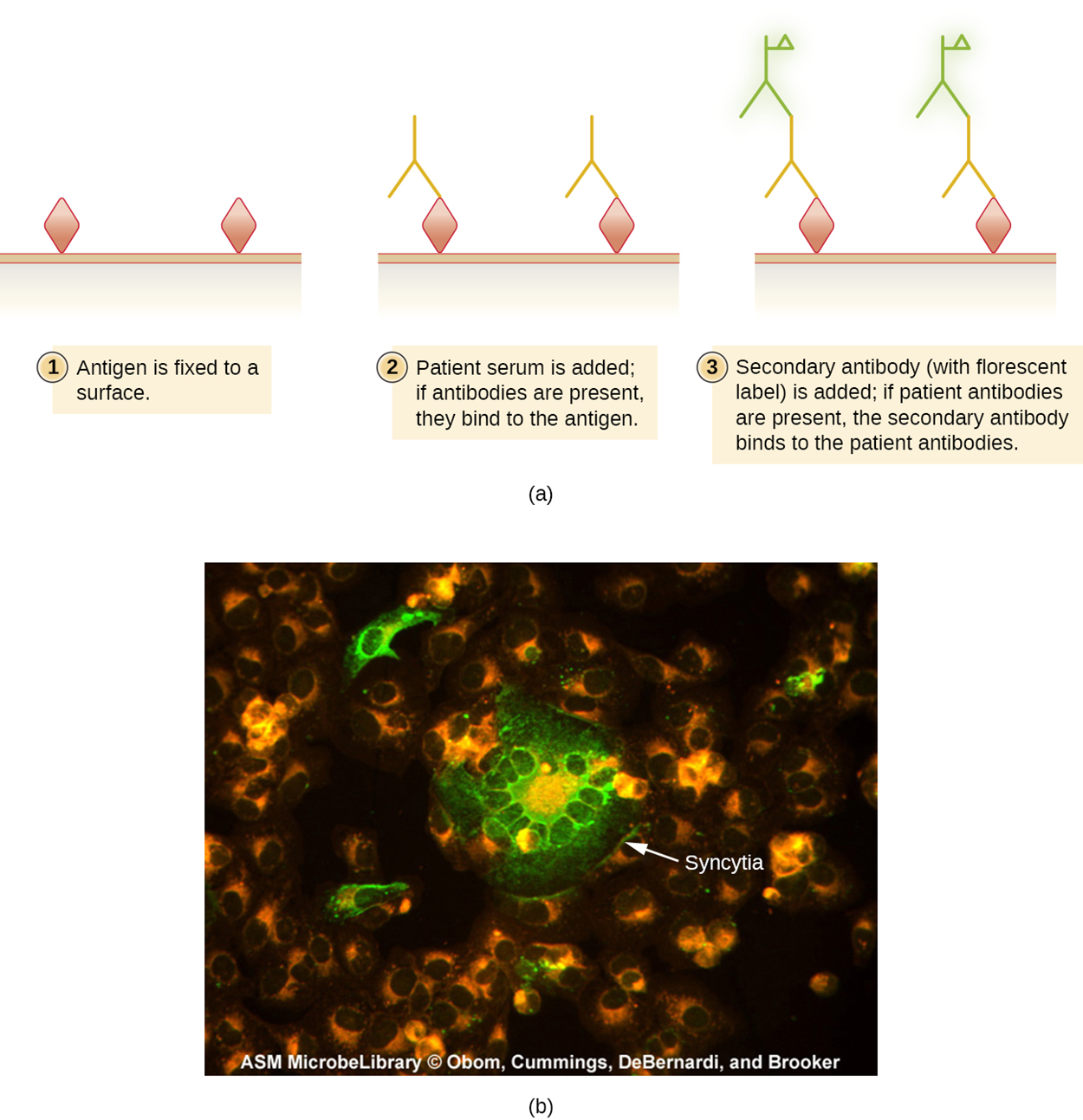
IFA tests can also be used to diagnose autoimmune diseases, which are often challenging to diagnose. The autoimmune disorder systemic lupus erythematosus (SLE) is characterized by elevated expression of antinuclear antibodies (ANA). These autoantibodies can be expressed against DNA-binding proteins and against DNA.
The steps and image below illustrate the test for ANA.
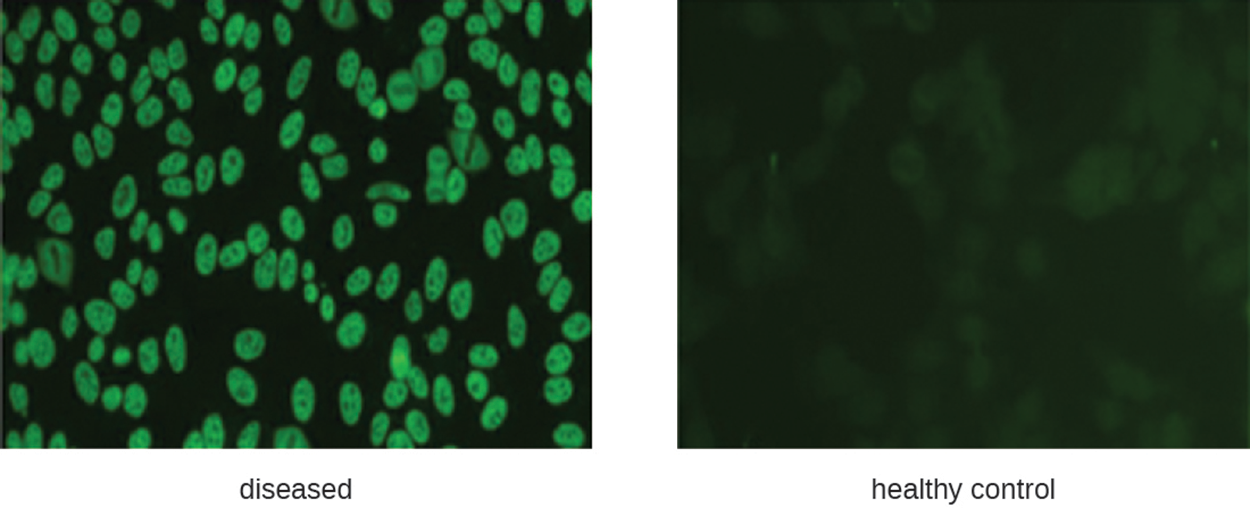
The left-hand micrograph below shows fluorescence around the nuclei of cells, which is characteristic of elevated ANA levels and possible SLE. The right-hand micrograph shows much less color in a slide from a healthy control.
Flow cytometry is an automated cell-counting system that detects fluorescing cells as they pass through a narrow tube one cell at a time. First, a mixed cell population is exposed to a fluorescently labeled mAb specific for the cell of interest. This causes target cells to be labeled with fluorescent antibodies. Some experiments use more than one mAb to mark more than one target. The cells are added to the flow cytometer through a narrow capillary that forces the cells to pass in single file and a laser is used to activate the fluorogen, which is detected by a fluorescence detector.
This can be useful for a variety of purposes, such as monitoring the immune system of patients with HIV. The level of CD4+ T cells can drop and increase the risk of opportunistic infections or even make it difficult for the patient to produce a functional adaptive immune response. Therefore, knowing CD4 T cell levels can be important in the care of these patients.
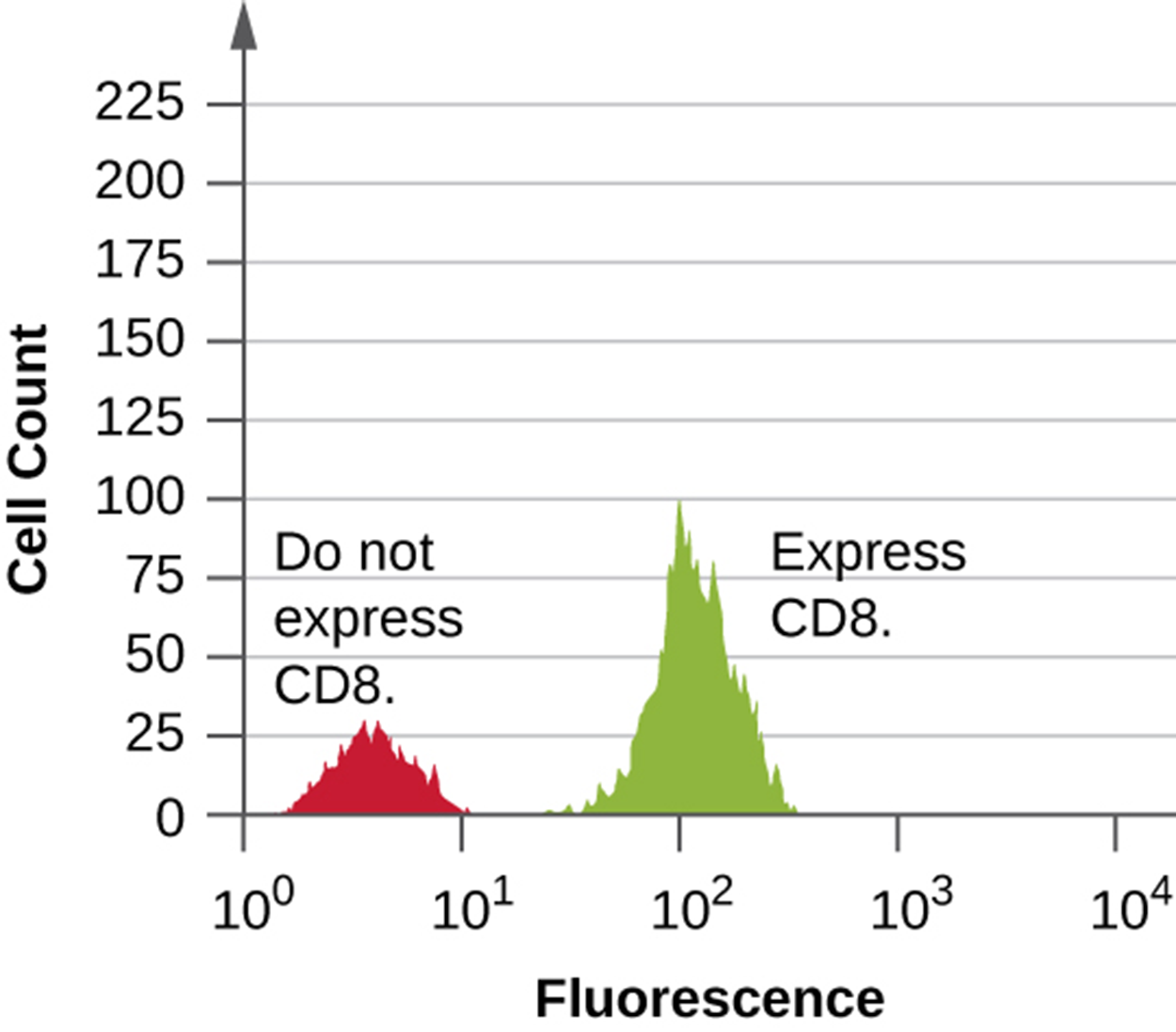
The histogram below shows data from flow cytometry performed on cells with an antibody attached to a fluorophore to detect CD8+ cells. In the histogram, there is a small left-hand peak formed by cells that do not fluoresce. The right-hand peak shows cells that fluoresce because they contain CD8 and therefore were bound by the fluorescently labeled antibody.
It is possible to modify the flow cytometer and immunofluorescence to sort cells from a single sample into purified subpopulations of cells. This is generally done for research purposes. This modified flow cytometer is called a fluorescence-activated cell sorter (FACS). Fluorescence detected by a device induces the device to put a charge on a droplet of the fluid transporting the cell and this charge is specific to the wavelength of fluorescent light. Therefore, cells given different charges because of different fluorescent markers can be sorted by their differing charges. An electrostatic detector moves the charged droplet containing the cell into a specific collecting vessel based on its charge.
This method can only be used to study isolated cells, such as white blood cells. Cells in tissue can only be studied in this way if first separated, so other approaches are generally easier. However, this technique is sometimes used to study tumor cells and other cells grown in laboratory cultures. Immunohistochemistry and immunocytochemistry are often preferred for studying cells in tissue.
The table below summarizes fluorescent antibody techniques discussed in this lesson, including mechanisms and examples.
| Fluorescent Antibody Techniques | ||
|---|---|---|
| Type of Assay | Mechanism | Examples |
| Direct fluorescent antibody (DFA) | Uses fluorogen–antibody conjugates to label bacteria from patient samples | Visualizing Legionella pneumophila from a throat swab |
| Indirect fluorescent antibody (IFA) | Detects disease-specific antibodies in patent serum | Diagnosing syphilis; detecting antinuclear antibodies (ANA) for lupus and other autoimmune diseases |
| Flow cytometry | Labels cell membranes with fluorogen–antibody conjugate markers excited by a laser; machine counts the cell and records the relative fluorescence | Counting the number of fluorescently labeled CD4+ or CD8+ cells in a sample |
| Fluorescence-activated cell sorter (FACS) | Form of flow cytometry that both counts cells and physically separates them into pools of high- and low-fluorescence cells | Sorting cancer cells |
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “MICROBIOLOGY.” ACCESS FOR FREE AT openstax.org/details/books/microbiology. LICENSE: CC ATTRIBUTION 4.0 INTERNATIONAL.