Table of Contents |
Electrons in atoms can exist only on discrete energy levels, not between them. It is said that the energy of an electron in an atom is quantized, meaning it can be equal only to certain specific values. It can jump from one energy level to another but not transition smoothly or stay between these levels.
The energy levels are labeled with an n value, where n = 1, 2, 3. Generally speaking, the energy of an electron in an atom is greater for greater values of n. The shells of an atom can be thought of as concentric circles radiating out from the nucleus.
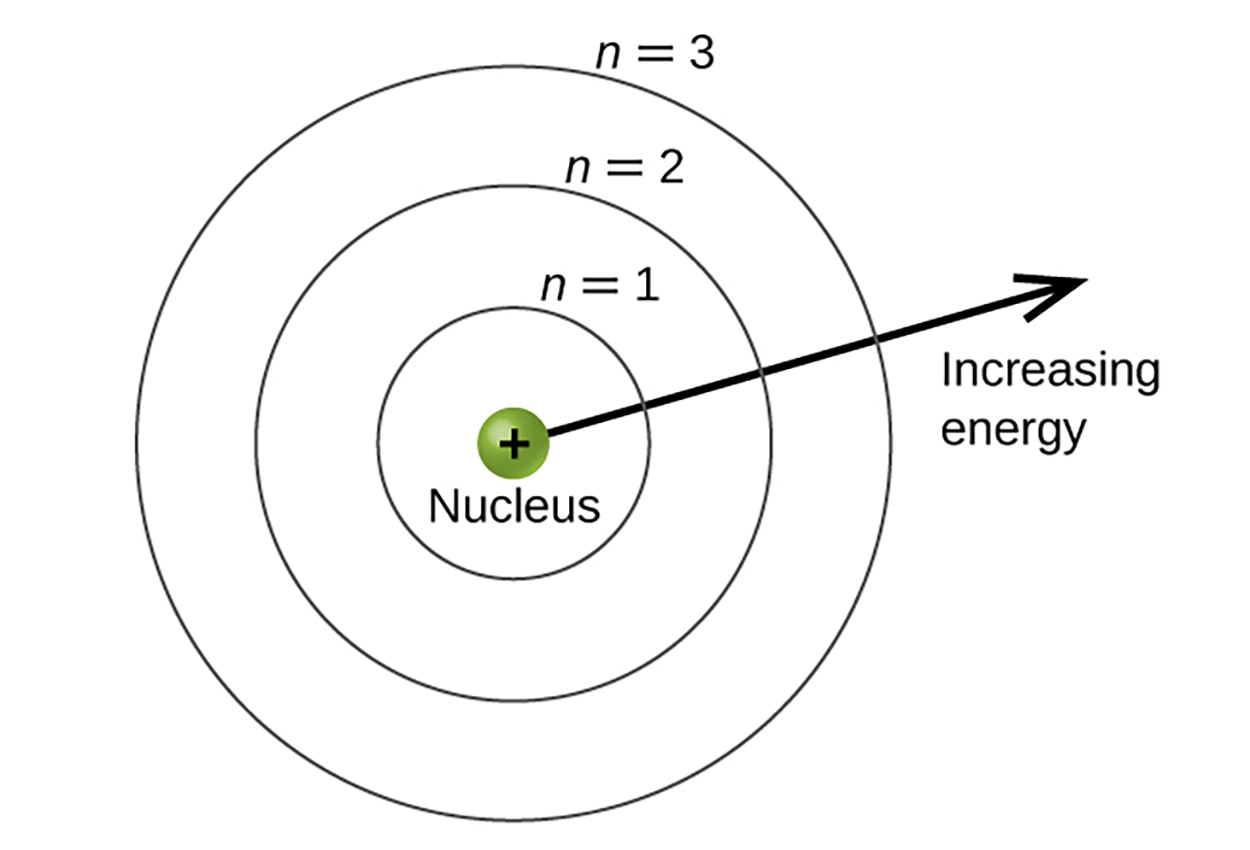
In a simplified view of this quantum mechanical model, the energy level can be referred to as an atomic orbital, which is a general region in an atom within which an electron is most probable to reside. Thus, n = 1 is the first atomic orbit and n = 2 is the second atomic orbit, and so forth.
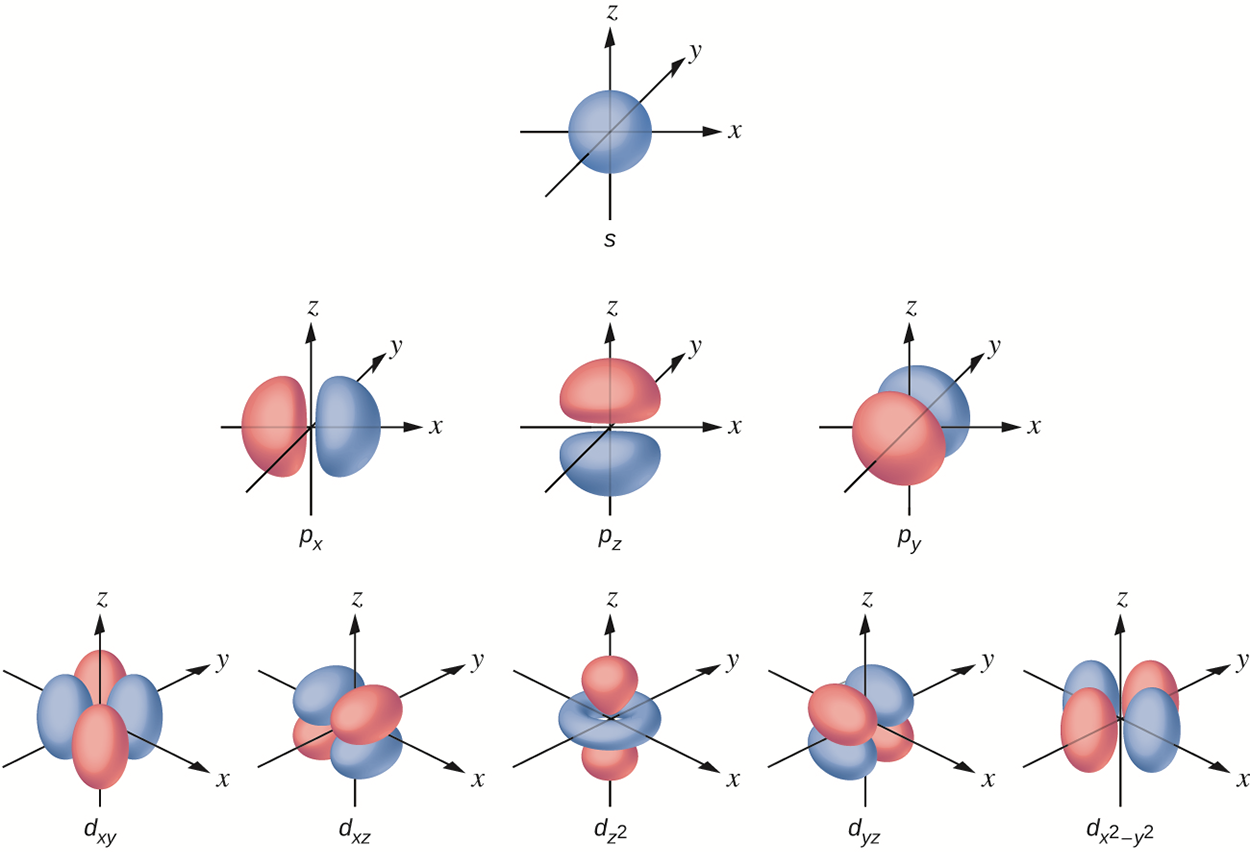
The energy level defines the general size and energy of the orbital. The subshell specifies the shape of the orbital. Orbitals of the same shape can be defined as a subshell. The main subshell orbitals are called s orbitals, p orbitals, and d orbitals. The s subshell is spherical and the p subshell has a dumbbell shape. The d-orbitals are more complex. These shapes represent the three-dimensional regions within which the electron is likely to be found.
Each s subshell has only one orbital, each p subshell has three equivalent orbitals, and each d subshell has five equivalent orbitals.
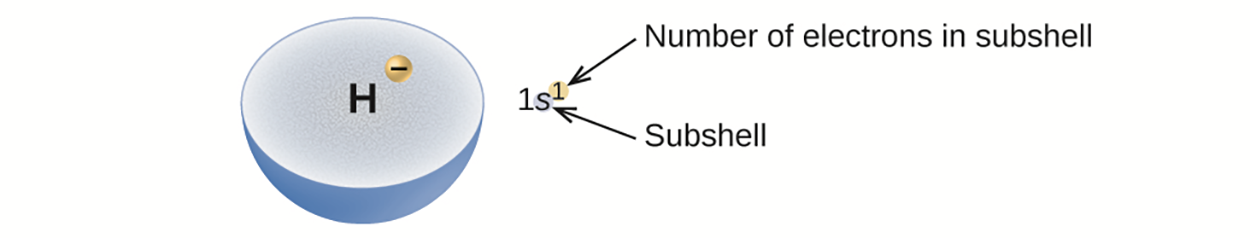
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information:
EXAMPLE
For example, the notation 2p (read "two–p–four") indicates four electrons in a p subshell with an energy level of 2. The notation 3d
(read "two–p–four") indicates four electrons in a p subshell with an energy level of 2. The notation 3d (read "three–d–eight") indicates eight electrons in the d subshell with an energy level of 3.
(read "three–d–eight") indicates eight electrons in the d subshell with an energy level of 3.
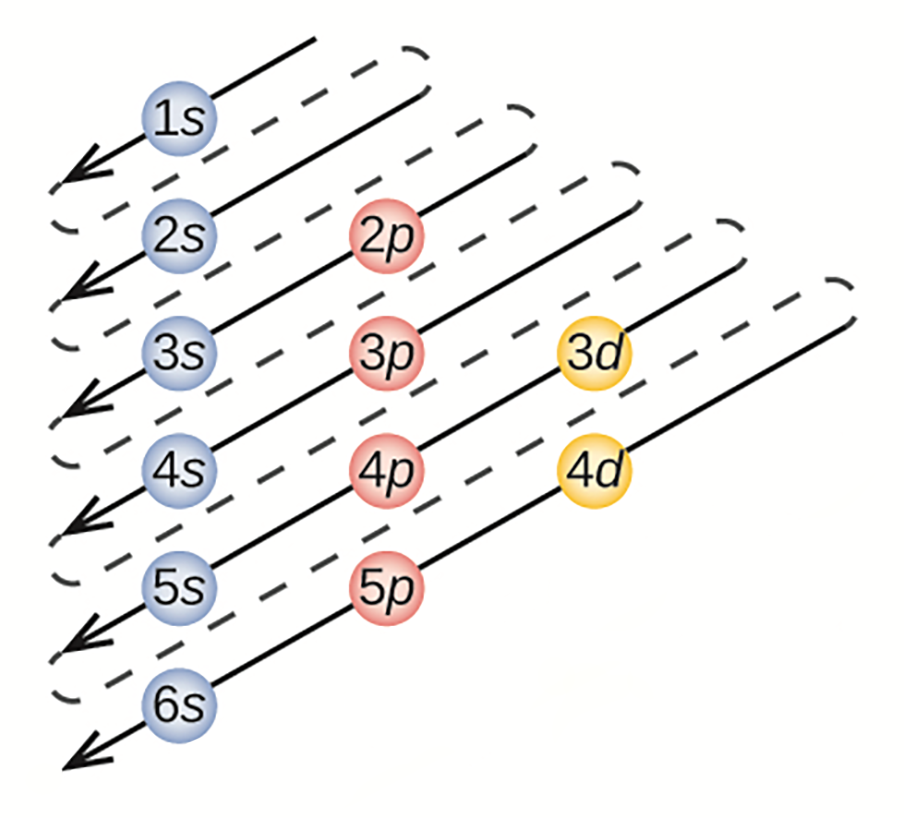
To determine the electron configuration for any particular atom, we can “build” the structures in the order of atomic numbers. This procedure is called the Aufbau principle, from the German word Aufbau (“to build up”).
Each added electron occupies the subshell of the lowest energy available. Electrons enter higher-energy subshells only after lower-energy subshells have been filled to capacity. The figure below illustrates the traditional way to remember the filling order for atomic orbitals.
Since the arrangement of the periodic table is based on the electron configurations, the periodic table in the image below provides an alternative method for determining the electron configuration. The filling order simply begins at hydrogen and includes each subshell as you proceed in the increasing Z-order. For example, after filling the 3p block up to Ar, we see the orbital will be 4s (K, Ca), followed by the 3d orbitals.
NOTE: We will not be discussing the two bottom rows of the periodic table, the lanthanides (Ce to Lu) and the actinides (Th-Lr) and we will not discuss f subshells.
Follow this link to WebElements to view an accessible version of the periodic table of elements. (Winter, 2021)
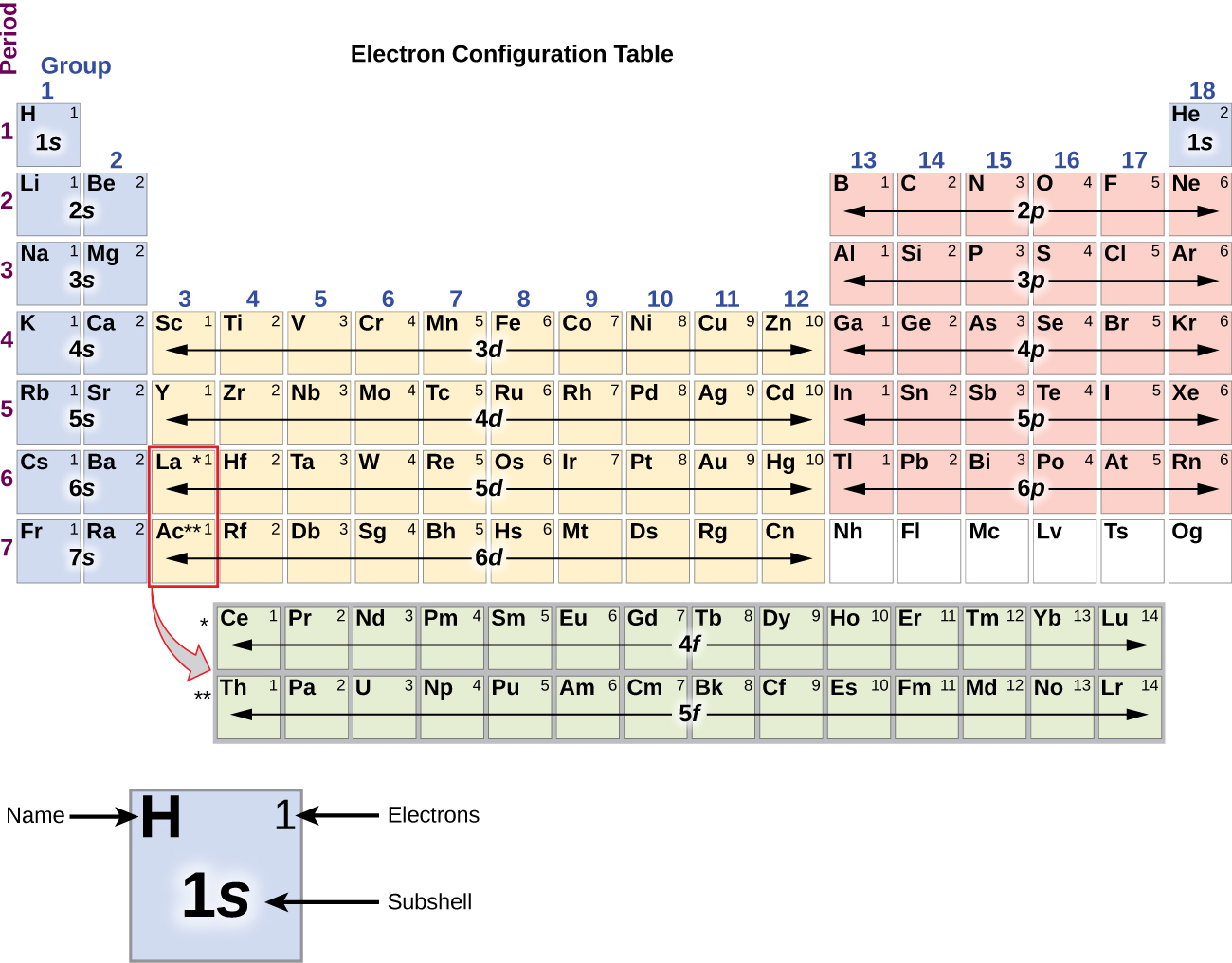
The image above of the periodic table shows the electron configuration for each subshell. By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table.
EXAMPLE
Using the periodic table above, determine the electron configuration of phosphorus. .
.
 2s
2s 2p
2p 3s
3s 3p
3p 4s
4s 3d
3d 4p
4p 5s
5s 4d
4d 5p
5p 6s
6s .
.
 needs to be included... therefore, phosphorus is 1s
needs to be included... therefore, phosphorus is 1s 2s
2s 2p
2p 3s
3s 3p
3p .
.
The Pauli exclusion principle states that no two electrons in the same atom can have exactly the same spin, which means that two electrons can share the same orbital only if they spin in opposite directions. We depict this as one arrow pointing up and one arrow pointing down. We can now construct the ground-state electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic table.
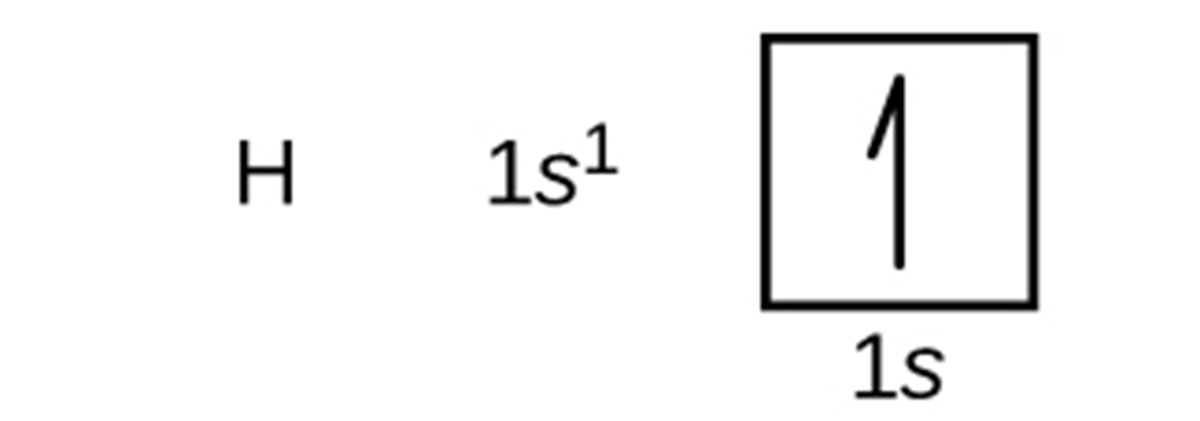
Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. We start with a single hydrogen atom (atomic number 1), which consists of one proton and one electron. The electron configuration and the orbital diagram are:
EXAMPLE
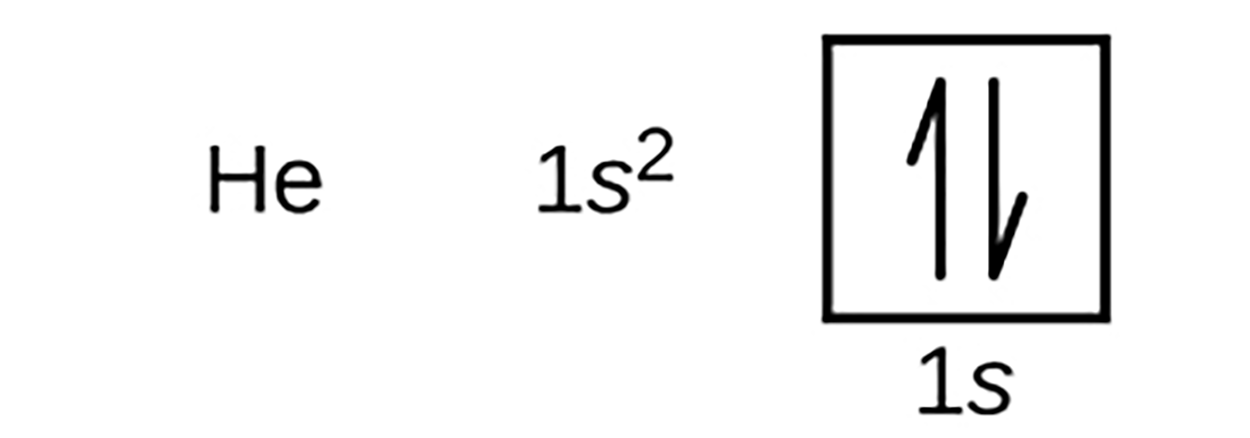
An atom of helium with an atomic number of 2, contains two electrons. According to the Pauli exclusion principle, the two arrows must point in opposite directions. The first energy level is completely filled in a helium atom. The electron configuration and orbital diagram of helium are:
EXAMPLE
An atom of lithium with an atomic number of 3, contains three electrons surrounding the nucleus. The first two electrons in lithium fill the 1s orbital. The remaining electron must occupy the orbital of the next lowest energy, the 2s orbital. Thus, the electron configuration and orbital diagram of lithium are:
EXAMPLE
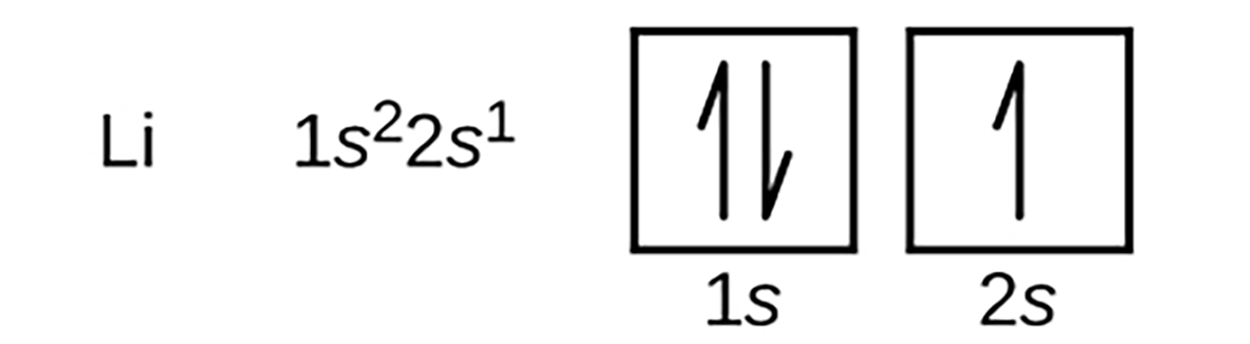
An atom of beryllium, with an atomic number of 4, contains four electrons surrounding the nucleus. The fourth electron fills the remaining space in the 2s orbital.
EXAMPLE
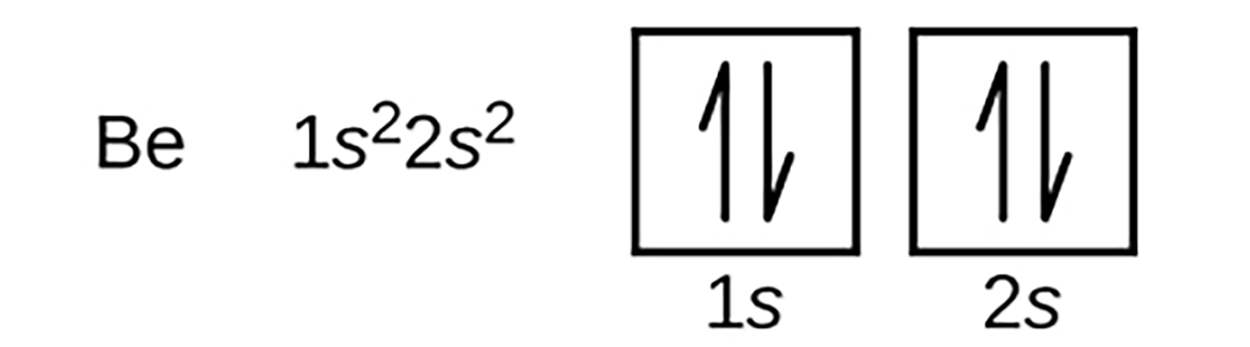
An atom of boron, with atomic number 5 contains five electrons. Energy level 1 is filled with two electrons and the remaining three electrons will fill in on the 2nd energy level. Because an s subshell can contain only two electrons, the fifth electron must occupy the next energy level, which will be a 2p orbital. There are three equivalent 2p orbitals and the electron can occupy any one of these p orbitals. When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling.
EXAMPLE
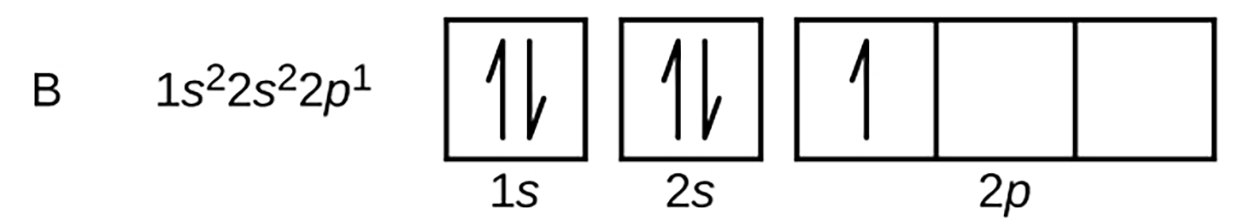
Carbon (atomic number 6) has six electrons. Four of them fill the 1s and 2s orbitals. The remaining two electrons occupy the 2p-subshell. We now have a choice of filling one of the 2p orbitals and pairing the electrons or of leaving the electrons unpaired in two different, but equivalent, p orbitals.
The orbitals are filled as described by Hund’s rule, which states that the lowest-energy configuration for an atom with electrons within a set of equivalent orbitals is that configuration having the maximum number of unpaired electrons. In other words, each orbital must have 1 electron before any orbital can have a pair of electrons in it.
EXAMPLE
The electron configuration and orbital diagram for carbon are: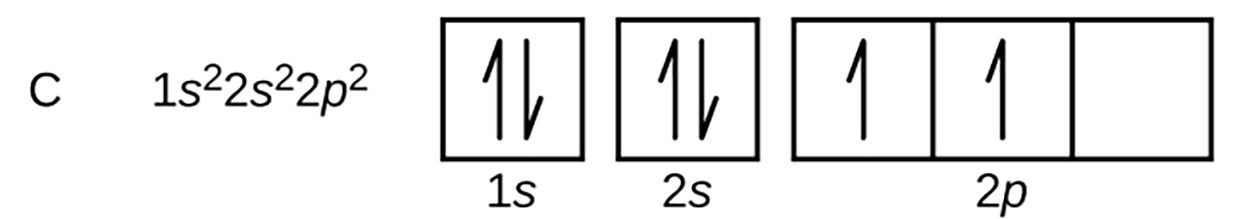
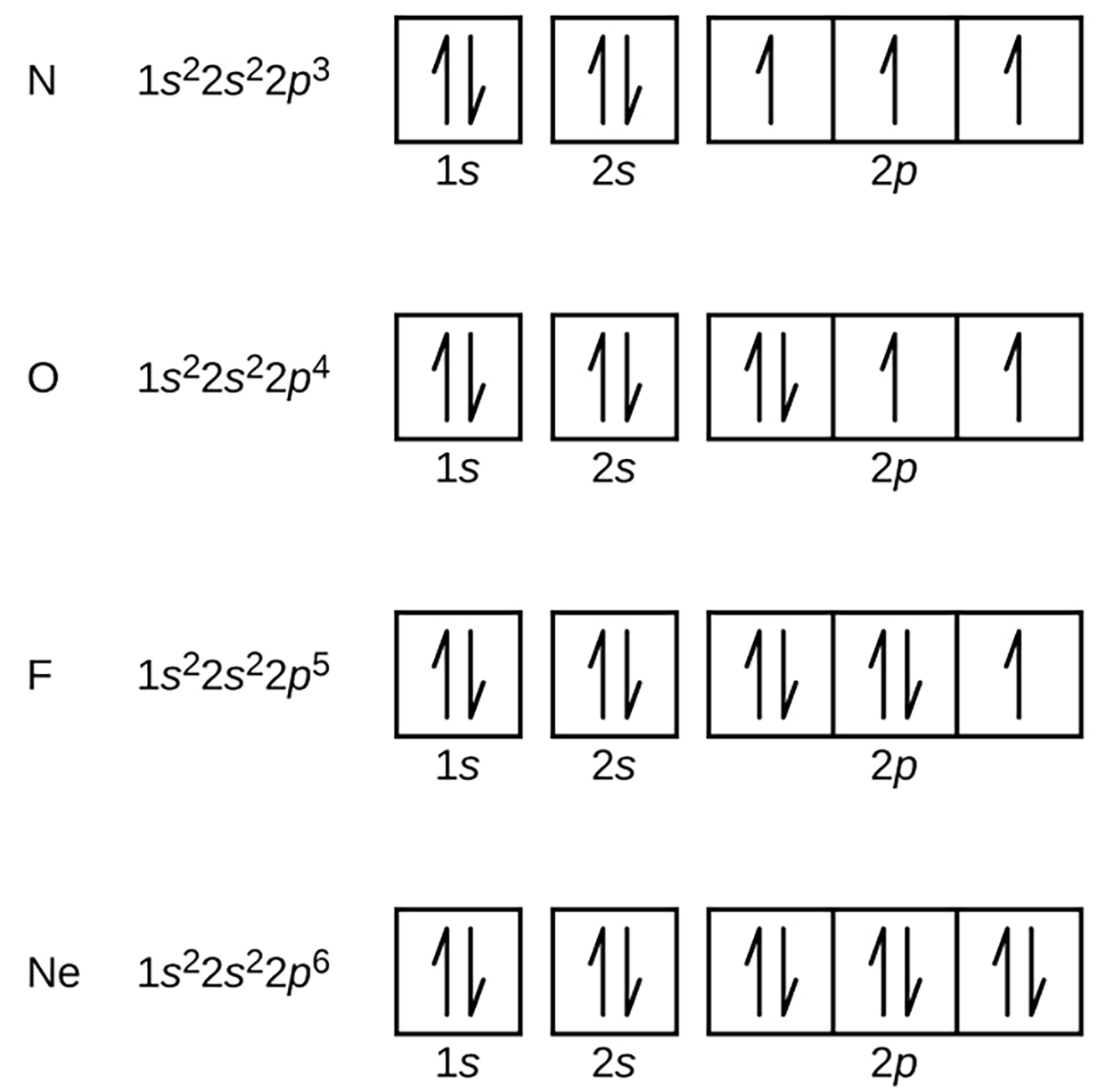
Nitrogen (atomic number 7) fills the 1s and 2s subshells and has one electron in each of the three 2p orbitals, in accordance with Hund’s rule.
Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single electron in each of the other two.
Fluorine (atomic number 9) has only one 2p orbital containing an unpaired electron.
All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the first and second energy levels are filled. The electron configurations and orbital diagrams of these four elements are:
The alkali metal sodium (atomic number 11) has one more electron than the neon atom. This electron must go into the lowest-energy subshell available, the 3s orbital, giving a 1s 2s
2s 2p
2p 3s
3s configuration. The electrons occupying the outermost shell orbital(s) (highest energy level) are called valence electrons. The electrons occupying the inner shell orbitals are called core electrons.
configuration. The electrons occupying the outermost shell orbital(s) (highest energy level) are called valence electrons. The electrons occupying the inner shell orbitals are called core electrons.
IN CONTEXT
Since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches the core electron configuration, along with the valence electrons in a condensed format. For sodium, the symbol [Ne] represents core electrons, (1s2s
2p
) and our abbreviated or condensed configuration is [Ne]3s
.
![Core electron configuration with a noble gas. The element Na has the electron configuration 1s22s22p63s1. The 1s22s22p6 portion of the electron configuration represents the core electrons, while the 3s1 portion of the electron configuration represents the valence electrons. The core electron portion (1s22s22p6) is the same electron configuration as Ne, so the electron configuration of Na can be abbreviated [Ne]3s1.](https://cdn.sophia.org/markup_pictures/12648/file/b421dcefdbebedbd261dfb8929168629.png)
The image above shows a core-abbreviated electron configuration (right) [Ne]3s replacing the core electrons (left) 1s
replacing the core electrons (left) 1s 2s
2s 2p
2p with the noble gas symbol 3s
with the noble gas symbol 3s , whose configuration matches the core electron configuration of the other element.
, whose configuration matches the core electron configuration of the other element.
Similarly, the abbreviated configuration of lithium can be represented as [He]2s . Writing the configurations in this way emphasizes the similarity of the configurations of lithium and sodium. Both atoms, which are in the alkali metal family, have only one electron in a valence s subshell outside a filled set of inner shells.
. Writing the configurations in this way emphasizes the similarity of the configurations of lithium and sodium. Both atoms, which are in the alkali metal family, have only one electron in a valence s subshell outside a filled set of inner shells.


Follow this link to WebElements to view an accessible version of the periodic table of elements. (Winter, 2021)
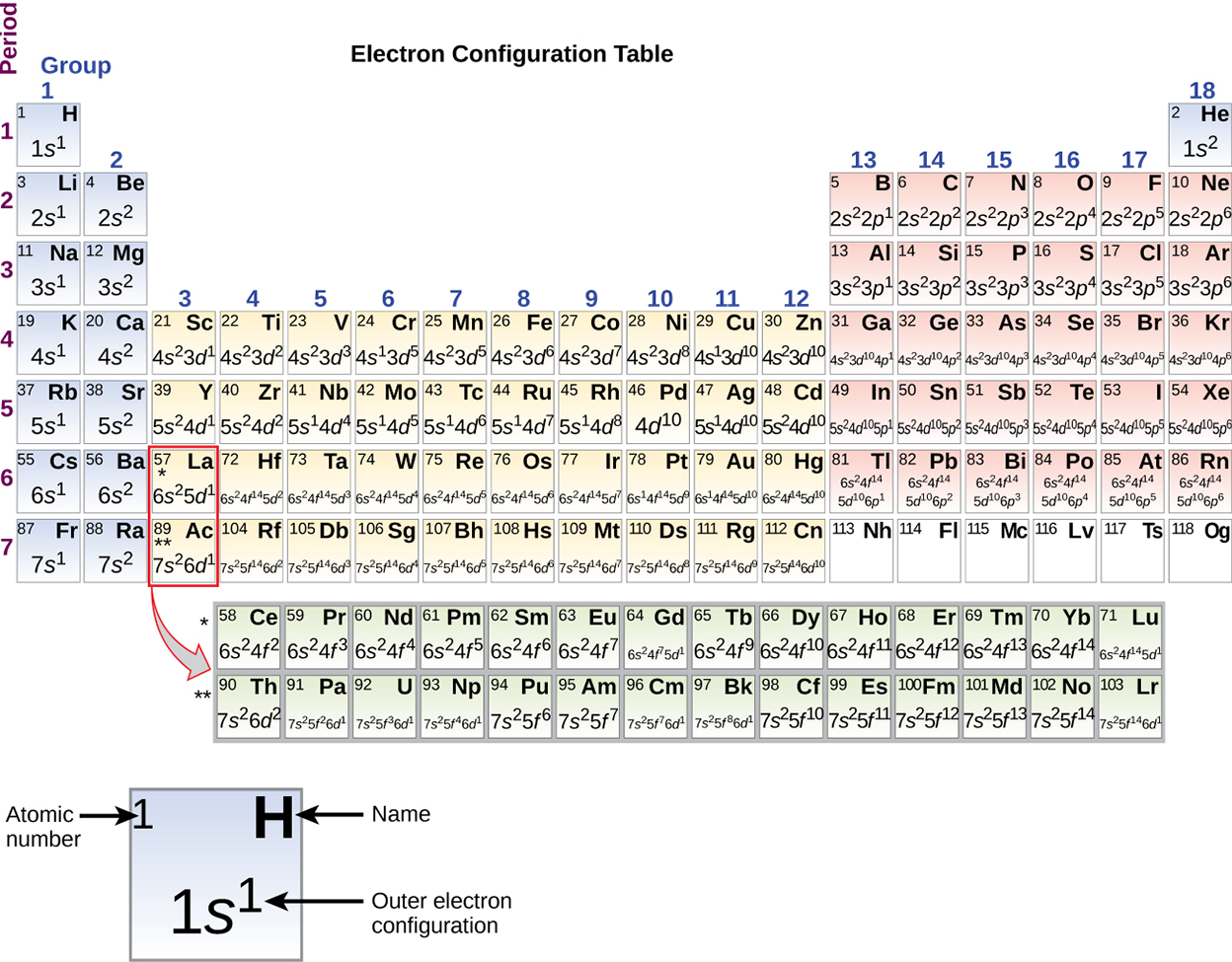
As described earlier, the periodic table arranges atoms based on increasing atomic numbers so that elements with the same chemical properties recur periodically. When their electron configurations are added to the table, we also see a periodic recurrence of similar electron configurations in the outer shells of these elements. Because they are in the outer shells of an atom, valence electrons play the most important role in chemical reactions. The outer electrons have the highest energy of the electrons in an atom and are more easily lost or shared than the core electrons. Valence electrons are also the determining factor in some physical properties of the elements.
Elements in any one group (or column) have the same number of valence electrons. The alkali metals lithium and sodium each has only one valence electron, the alkaline earth metals beryllium and magnesium each has two, and the halogens fluorine and chlorine each has seven valence electrons. The similarity in chemical properties among elements of the same group occurs because they have the same number of valence electrons. It is the loss, gain, or sharing of valence electrons that defines how elements react.
IN CONTEXT
It is important to remember that the periodic table was developed on the basis of the chemical behavior of the elements, well before any idea of their atomic structure was available. Now we can understand why the periodic table has the arrangement it has—the arrangement puts elements whose atoms have the same number of valence electrons in the same group.
Ions are formed when atoms gain or lose electrons. A cation (positively charged ion) forms when one or more electrons are removed from a parent atom. For main group elements, the electrons that were added last are the first electrons removed. An anion (negatively charged ion) forms when one or more electrons are added to a parent atom. The added electrons fill in the order predicted by the Aufbau principle.
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “CHEMISTRY: ATOMS FIRST 2E”. ACCESS FOR FREE AT Chemistry: Atoms First 2e. LICENSE: CREATIVE COMMONS ATTRIBUTION 4.0 INTERNATIONAL
REFERENCES
Winter, M. (2021). The periodic table of the elements. The periodic table of the elements by WebElements. https://webelements.com/.