Table of Contents |
In this lesson, you will learn about different types of tests that use antibodies to detect the presence of antigens. These tests, called enzyme immunoassays (EIAs), are carried out in microtiter plates or in vivo and share a common characteristic: they use an antibody molecule with a variable region that binds to a specific antigen and a constant region that binds to an enzyme. The enzyme is important because it can undergo a reaction to show the presence of the antibody, allowing rapid visualization and/or quantification of test results.
Chromogens are colorless molecules that can be converted into a colored end product. EIAs often use enzymes that react with chromogens. Alkaline phosphatase and horseradish peroxidase are commonly used.
In some cases, a fluorogen is the substrate for the enzyme. Fluorogens are nonfluorescent molecules that can be converted by the enzyme into a fluorescent form that can be detected using a spectrophotometer or a fluorescence microscope. When EIAs use fluorogens, they are called fluorescent enzyme immunoassays (FEIAs).
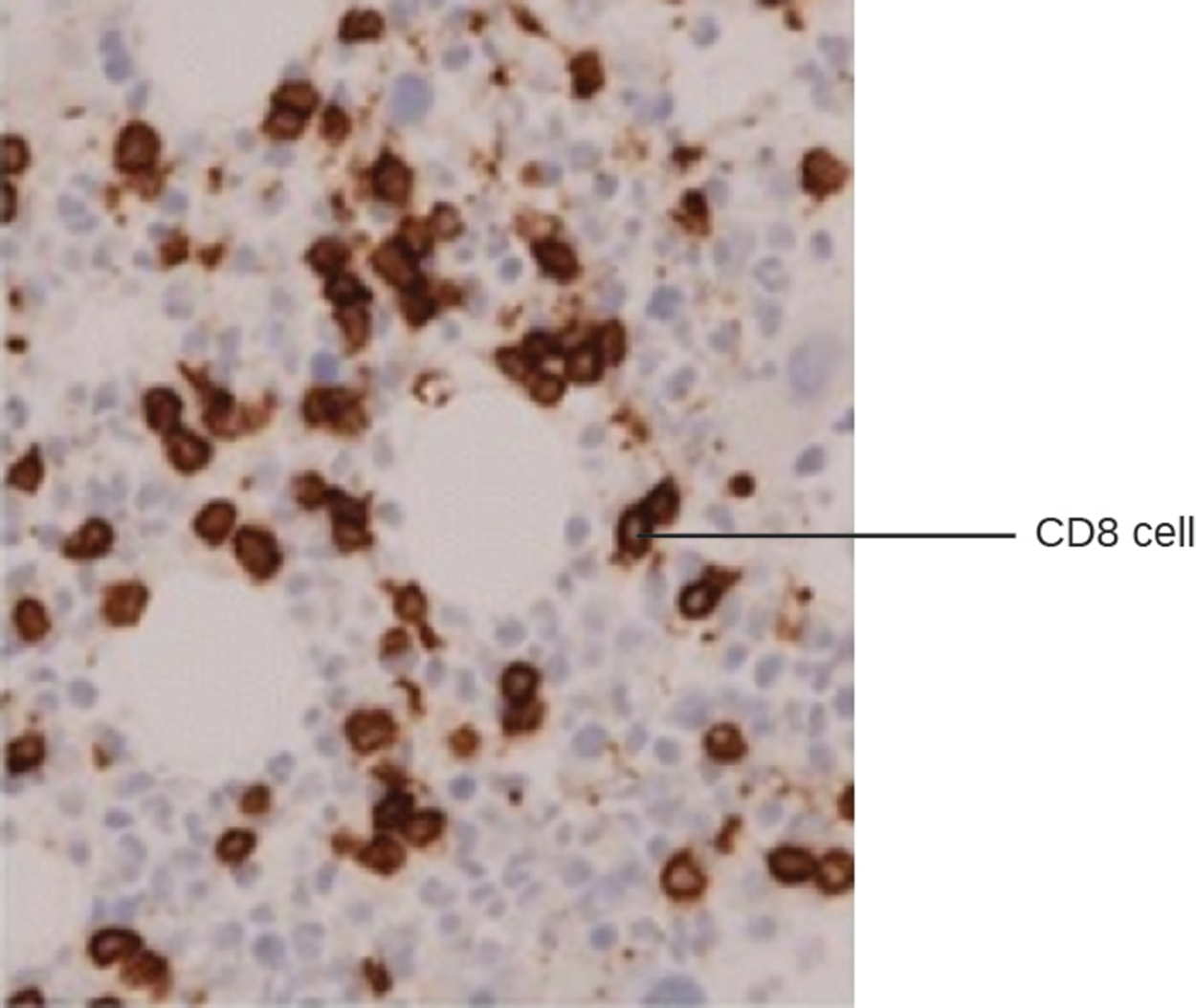
EIA can be used for immunostaining. The antibody–enzyme conjugate enhances microscopy by making antigens of interest more visible. The micrograph below shows an example of immunohistochemistry (IHC), which is similar to immunostaining but used to visualize different cell types. In the micrograph, an mAb against CD8 was used to stain CD8+ cells in a section of tissue. This allows the number of CD8 cells to be counted, located, and compared with the number of CD8 cells in other tissues.
Immunocytochemistry (ICC) is another type of immunostaining that is similar to IHC but is performed after the extracellular matrix has been stripped away and alcohol has been added to make the cell membrane permeable to antibodies. Antibodies can therefore enter the cell to bind to specific targets such as organelles. Some ICC techniques use EIA and others use fluorescent molecules in place of enzymes (a fluorescent immunoassay).
Enzyme-linked immunosorbent assays (ELISAs) are very commonly used examples of EIAs. There are three major types of ELISAs:
Direct ELISA
The image below shows an example of a direct ELISA test that produces a color change, indicating a positive result (i.e., presence of the antigen of interest).
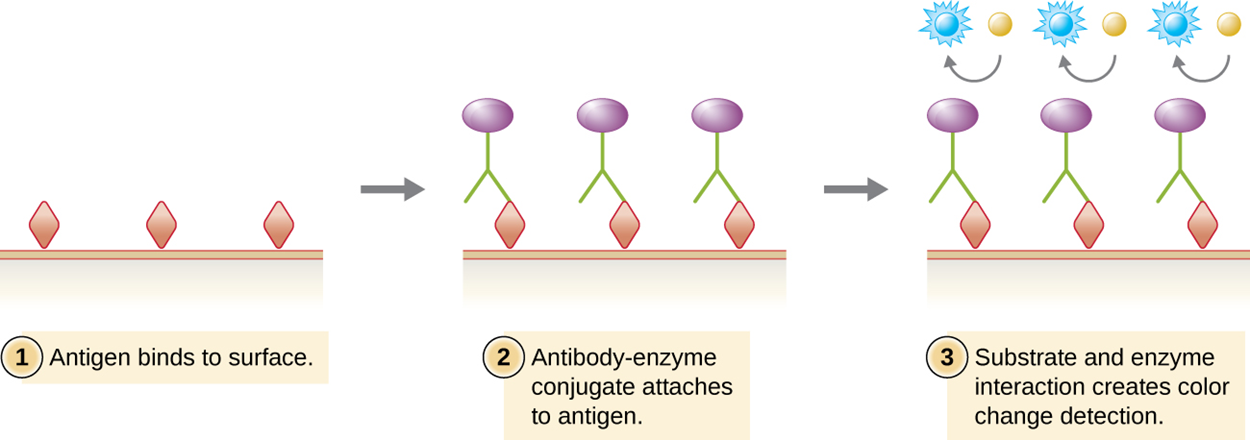
Direct ELISA tests are rapid and relatively simple, but other types of ELISA tests are sometimes preferred for various reasons (such as higher sensitivity).
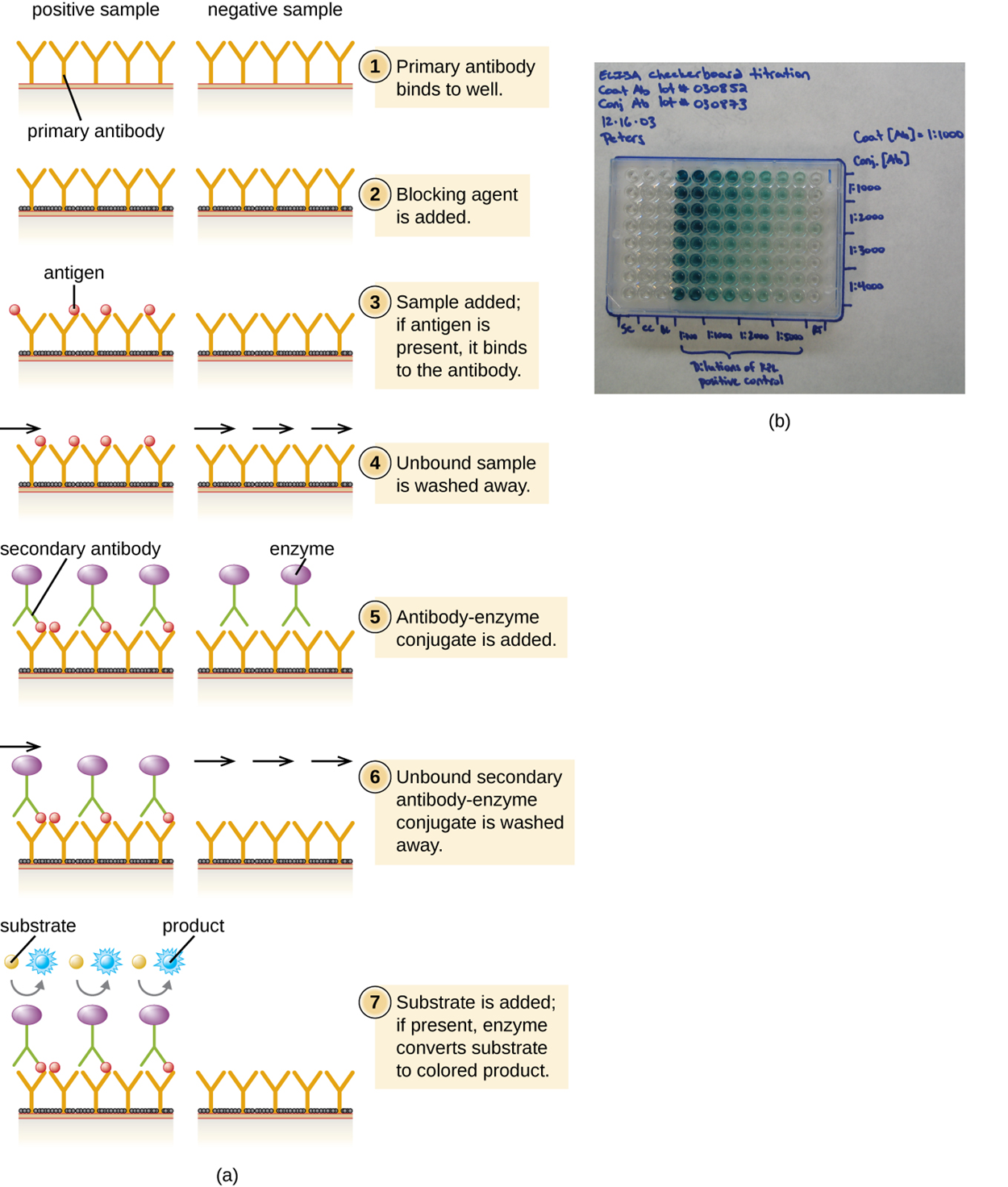
The sandwich ELISA test uses antibodies to quantify the exact amount of an antigen present in a sample. The test is summarized in the steps and image below.
The figure below presents a simplified version of the steps provided above. Note that the figure does not include preparation of a standardized curve, which adds steps above.
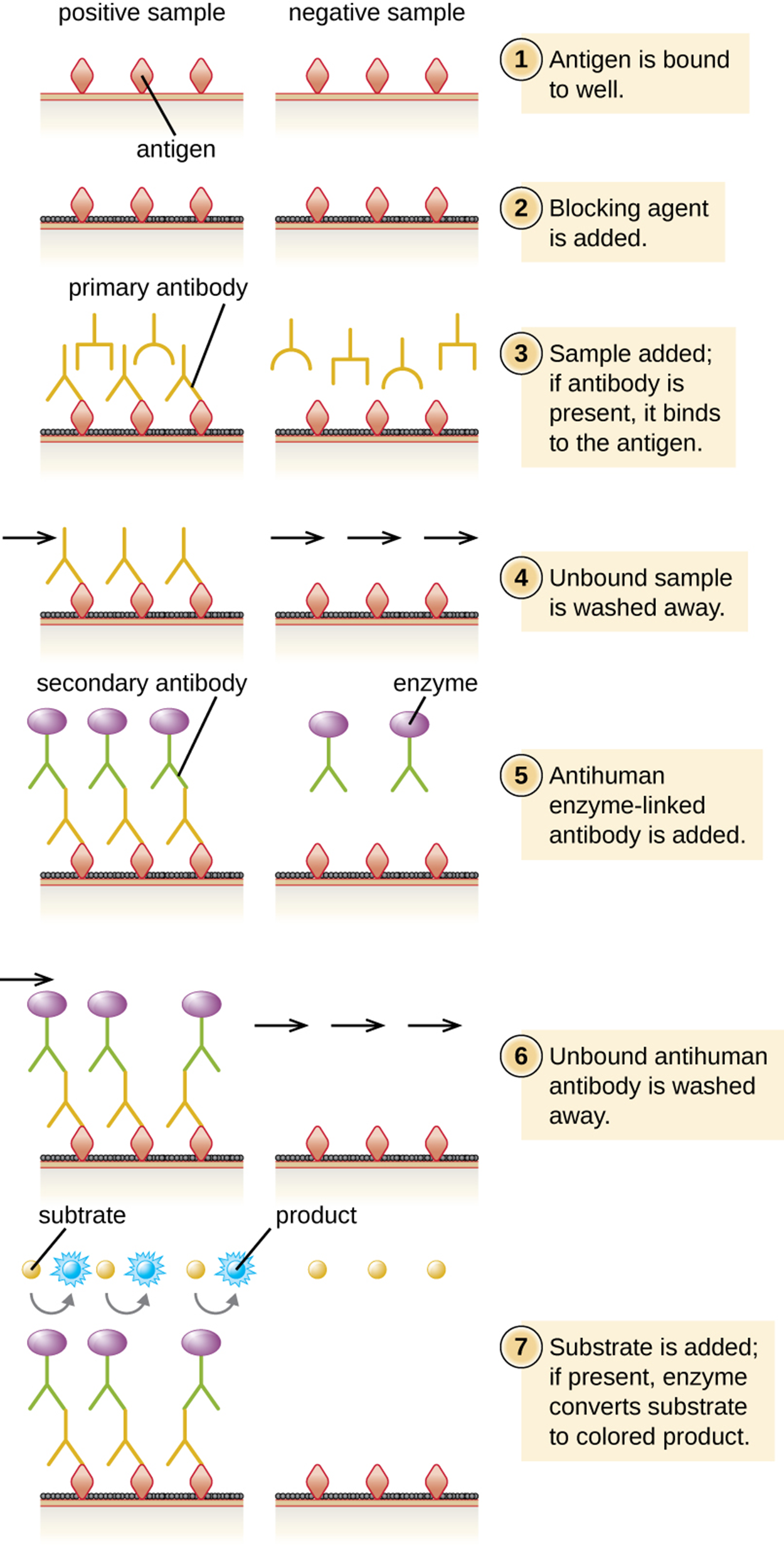
Indirect ELISA is used to quantify antigen-specific antibody instead of antigen. Therefore, it is useful to detect antibodies against a variety of pathogens. However, it has some important differences from direct ELISA. The first step is to attach a known antigen (not antibody) to the bottom of the microtiter plate wells. After unbound sites are blocked, patient serum is added and any primary antibodies present bind to antigen. After washing away unbound proteins, the secondary antibody with its conjugated enzyme is added. This antibody targets the first antibody. The intensity of color produced by the conjugated enzyme–chromogen reaction is used to quantify how much antigen-specific antibody is present in the patient’s serum.
Positive tests must be confirmed, especially as cross-reactivity may occur. This is often accomplished using immunoblots to identify the presence of specific peptides or tests to identify nucleic acids. The steps of indirect ELISA are summarized in the steps and image below.
Immunofiltration is used to detect or quantify antigens or antibodies that are present in very low quantities. In this process, fluid is passed through a porous membrane into an absorbent pad. The pad has either antigens or antibodies attached to capture antibodies or antigens, respectively.
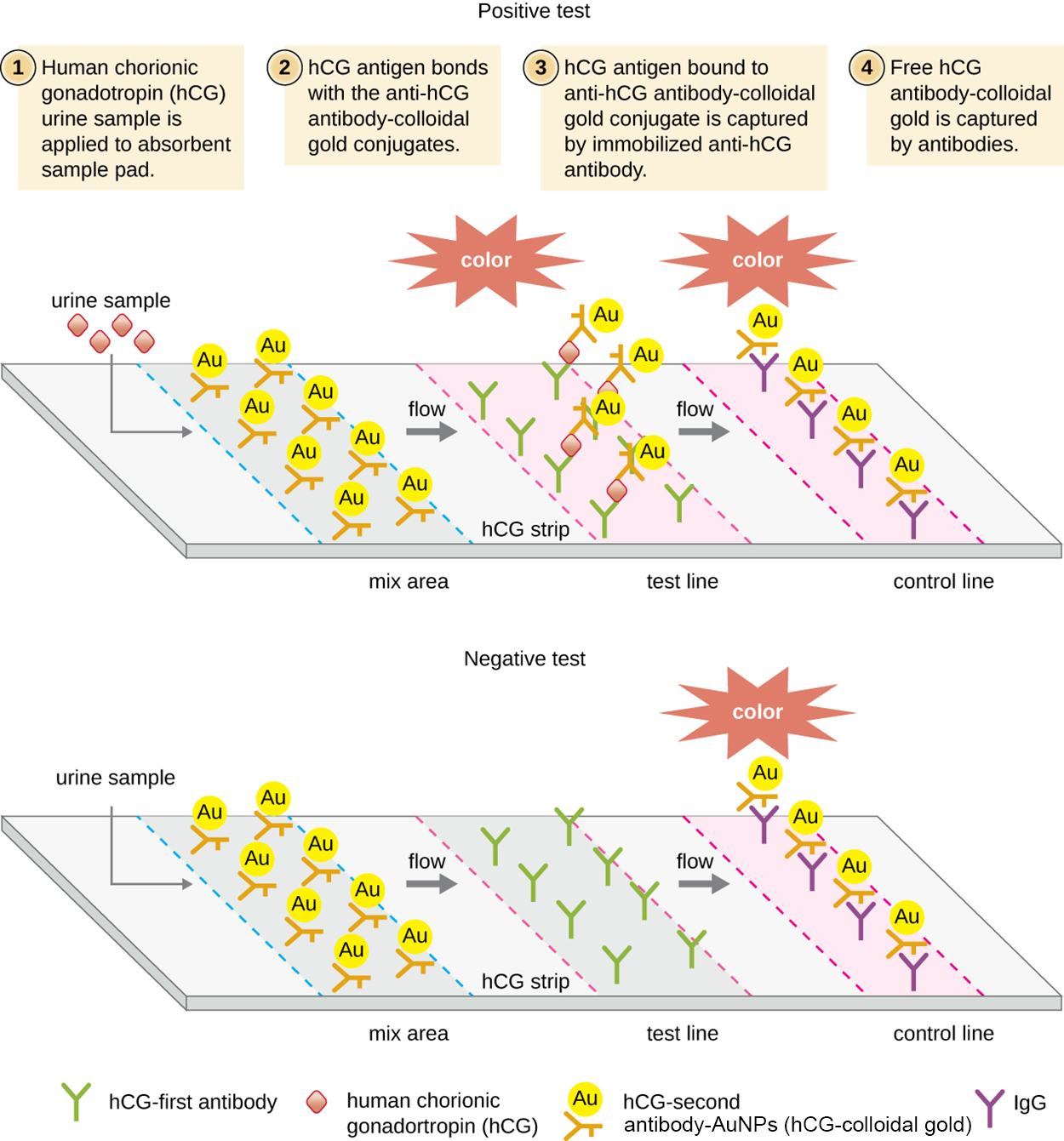
Immunochromatographic assays (also called lateral flow tests) have been developed using an adaptation of immunofiltration. In these tests, fluids are applied to an absorbent pad on a test strip. Fluid flows through capillary action and moves through a strip of beads with antibodies attached to their surfaces. The fluid in the sample hydrates the reagents in the strip. Antibody-coated beads made of latex or tiny gold particles bind to antigens in the test fluid as it passes across them. The antibody–antigen complexes then flow over a second strip that has immobilized antibody against the antigen; this strip retains beads that have bound antigen. A third control strip binds any beads regardless of the presence or absence of antigen. Color develops at the test lines if the test is positive. If the test was performed correctly but the antigen is absent, then color only develops at the control line.
These tests are commonly used for in-home pregnancy tests as well as the TORCH test, which is used to screen pregnant women or newborns for a range of infections called the TORCH complex, which includes toxoplasmosis, others (syphilis, hepatitis B), rubella, cytomegalovirus, and herpes simplex.
These tests take advantage of antibody sandwiches, which provide sensitivity and specificity. They are fast and inexpensive, even though they are not as quantitative as ELISA.
The photo below shows a positive pregnancy test, which is an example of a lateral flow test. Two lines have formed through the process described above.
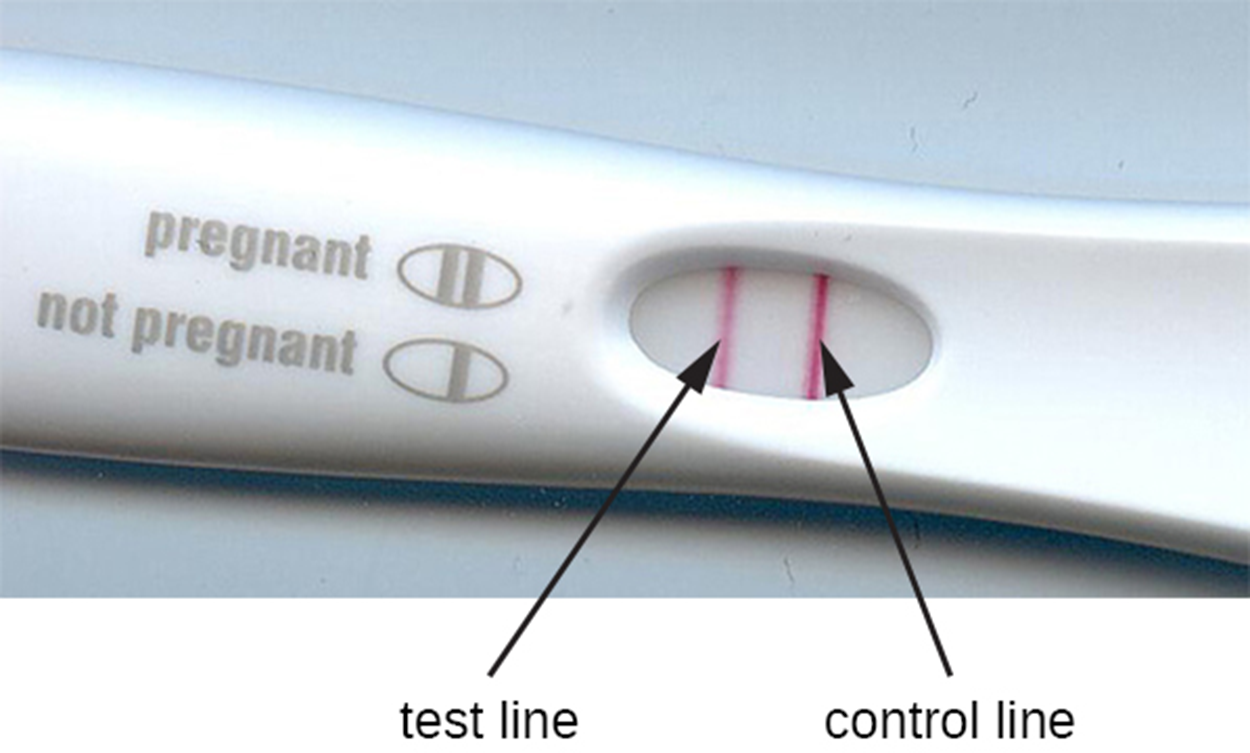
As an example of how immunochromatographic assays (lateral flow tests) work, the steps and image below describe a human pregnancy test.
The table below summarizes the mechanisms and procedures of the assays discussed in this lesson including examples.
| Immunoblots and Enzyme Immunoassays | |||
|---|---|---|---|
| Type of Assay | Mechanism | Specific Procedures | Examples |
| Immunoblots | Uses enzyme–antibody conjugates to identify specific proteins that have been transferred to an absorbent membrane | Western blot: Detects the presence of a particular protein | Detecting the presence of HIV peptides (or peptides from other infectious agents) in patient sera |
| Immunostaining | Uses enzyme–antibody conjugates to stain specific molecules on or in cells | Immunohistochemistry: Used to stain specific cells in a tissue | Stain for presence of CD8+ cells in host tissue |
| Enzyme-linked immunosorbent assay (ELISA) | Uses enzyme–antibody conjugates to quantify target molecules | Direct ELISA: Uses a single antibody to detect the presence of an antigen | Detection of HIV antigen p24 up to one month after being infected |
| Indirect ELISA: Measures the amount of antibody produced against an antigen | Detection of HIV antibodies in serum | ||
| Immunochromatographic (lateral flow) assays | Techniques use the capture of flowing, color-labeled antigen–antibody complexes by fixed antibody for disease diagnosis | Lateral flow assays for pregnancy, COVID-19 antigens, and others | Detection of antibodies for various pathogens in patient sera (e.g., rapid strep, malaria dipstick) |
| Pregnancy test detecting human chorionic gonadotrophin in urine | |||
| Detection of COVID-19 antigens | |||
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “MICROBIOLOGY.” ACCESS FOR FREE AT openstax.org/details/books/microbiology. LICENSE: CC ATTRIBUTION 4.0 INTERNATIONAL.
REFERENCES
Jillian, K. MS, RD. (2022, December 16). Rapid COVID-19 Tests: When to Use Them and How They Work. Healthline. www.healthline.com/health-news/rapid-covid-19-tests-when-to-use-them-and-how-they-work
Ng, Q. X., Lim, Y. L., Han, M. X., Teoh, S. E., Thumboo, J., & Tan, B. H. (2022). The Performance of Lateral Flow Tests in the Age of the Omicron: A Rapid Systematic Review. Life (Basel, Switzerland), 12(11), 1941. doi.org/10.3390/life12111941