Table of Contents |
Normal arterial blood pH is restricted to a very narrow range of 7.35 to 7.45. A person who has a blood pH below 7.35 is considered to be in acidosis (actually, “physiological acidosis,” because blood is not truly acidic until its pH drops below 7), and a continuous blood pH below 7.0 can be fatal. Acidosis has several symptoms, including headache and confusion, and the individual can become lethargic and easily fatigued. A person who has a blood pH above 7.45 is considered to be in alkalosis, and a pH above 7.8 is fatal.
Some symptoms of alkalosis include:
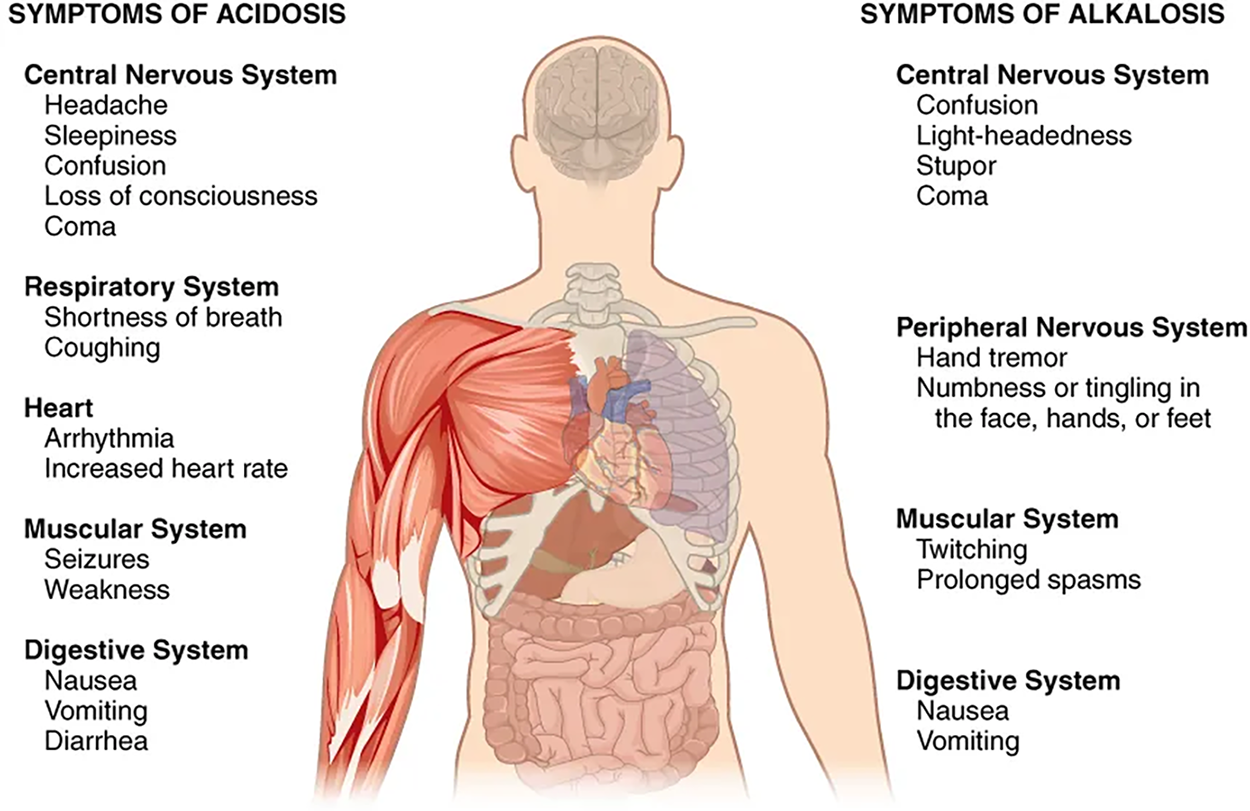
The major disorders associated with abnormal blood pH levels are described below.
Metabolic acidosis occurs when the blood is too acidic (pH below 7.35) due to too little bicarbonate, a condition called primary bicarbonate deficiency. At the normal pH of 7.40, the ratio of bicarbonate to carbonic acid buffer is 20:1. If a person’s blood pH drops below 7.35, then the person is in metabolic acidosis. The most common cause of metabolic acidosis is the presence of organic acids or excessive ketone bodies in the blood. The table below lists some other causes of metabolic acidosis. In this table, asterisks (*) indicate metabolites from ingested chemicals.
| Cause | Metabolite |
|---|---|
| Diarrhea | Bicarbonate |
| Uremia | Phosphoric, sulfuric, and lactic acids |
| Diabetic ketoacidosis | Increased ketone bodies |
| Strenuous exercise | Lactic acid |
| Methanol | Formic acid* |
| Paraldehyde | β-Hydroxybutyric acid* |
| Isopropanol | Propionic acid* |
| Ethylene glycol | Glycolic acid, and some oxalic and formic acids* |
| Salicylate/aspirin | Sulfasalicylic acid (SSA)* |
The first three of the nine causes of metabolic acidosis listed above are medical (or unusual physiological) conditions.
EXAMPLE
Metabolic acidosis can also result from uremia, which is the retention of urea and uric acid, and from diabetic ketoacidosis, wherein an excess of ketone bodies is present in the blood. Strenuous exercise can cause temporary metabolic acidosis because of the production of lactic acid.Metabolic alkalosis is the opposite of metabolic acidosis. It occurs when the blood is too alkaline (pH above 7.45) and is due to too much bicarbonate (called primary bicarbonate excess).
A transient excess of bicarbonate in the blood can follow ingestion of excessive amounts of bicarbonate, citrate, or antacids for conditions such as stomach acid reflux—known as heartburn. Cushing’s disease, which is the chronic hypersecretion of adrenocorticotropic hormone (ACTH) by the anterior pituitary gland, can cause chronic metabolic alkalosis. The oversecretion of ACTH results in elevated aldosterone levels and an increased loss of potassium by urinary excretion. Other causes of metabolic alkalosis include the loss of hydrochloric acid from the stomach through vomiting, potassium depletion due to the use of diuretics for hypertension, and the excessive use of laxatives.
Respiratory acidosis occurs when the blood is overly acidic due to an excess of CO₂ in the blood. Respiratory acidosis can result from anything that interferes with respiration, such as pneumonia, emphysema, or congestive heart failure.
Respiratory alkalosis occurs when the blood is overly alkaline due to a deficiency in CO₂ levels in the blood. This condition usually occurs when too much CO₂ is exhaled from the lungs, as occurs in hyperventilation, which is breathing that is deeper or more frequent than normal. An elevated respiratory rate leading to hyperventilation can be due to extreme emotional upset or fear, fever, infections, hypoxia (e.g., at high altitudes), or abnormally high levels of catecholamines, such as epinephrine and norepinephrine. Surprisingly, aspirin overdose—salicylate toxicity—can result in respiratory alkalosis as the body tries to compensate for initial acidosis.
| Term | Pronunciation | Audio File |
|---|---|---|
| Acidosis | ac·i·do·sis |
|
| Alkalosis | al·ka·lo·sis |
|
Various compensatory mechanisms exist to maintain blood pH within a narrow range, including buffers, respiration, and renal mechanisms. Although compensatory mechanisms usually work very well, when one of these mechanisms is not working properly (like kidney failure or respiratory disease), they have their limits. If the pH and bicarbonate to carbonic acid ratio are changed too drastically, the body may not be able to compensate. Moreover, extreme changes in pH can denature proteins. Extensive damage to proteins in this way can result in disruption of normal metabolic processes, serious tissue damage, and ultimately death.
Respiratory compensation for metabolic acidosis increases the respiratory rate to drive off CO₂ and readjust the bicarbonate to carbonic acid ratio to the 20:1 level. This adjustment can occur within minutes. Respiratory compensation for metabolic alkalosis is not as adept as its compensation for acidosis. The normal response of the respiratory system to elevated pH is to increase the amount of CO₂ in the blood by decreasing the respiratory rate to conserve CO₂. However, there is a limit to the decrease in respiration that the body can tolerate. Hence, the respiratory route is less efficient at compensating for metabolic alkalosis than for acidosis.
Metabolic and renal compensation for respiratory diseases that can create acidosis revolves around the conservation of bicarbonate ions. In cases of respiratory acidosis, the kidney increases the conservation of bicarbonate and secretion of H⁺ through the exchange mechanism discussed earlier. These processes increase the concentration of bicarbonate in the blood, reestablishing the proper relative concentrations of bicarbonate and carbonic acid.
In cases of respiratory alkalosis, the kidneys decrease the production of bicarbonate and reabsorb H⁺ from the tubular fluid. These processes can be limited by the exchange of potassium by the renal cells, which use a K⁺–H⁺ exchange mechanism (antiporter).
Lab tests for pH, CO₂ partial pressure (PCO₂), and HCO₃⁻ can identify acidosis and alkalosis, indicating whether the imbalance is respiratory or metabolic, and the extent to which compensatory mechanisms are working. The blood pH value indicates whether the blood is in acidosis, the normal range, or alkalosis. The PCO₂ and total HCO₃⁻ values aid in determining whether the condition is metabolic or respiratory and whether the patient has been able to compensate for the problem.
The table below lists the conditions and laboratory results that can be used to classify these conditions. Metabolic acid–base imbalances typically result from kidney disease, and the respiratory system usually responds to compensate.
| pH | PCO₂ | Total HCO₃⁻ | |
|---|---|---|---|
| Metabolic acidosis | ↓ | Normal, then ↓ | ↓ |
| Respiratory acidosis | ↓ | ↑ | Normal, then ↑ |
| Metabolic alkalosis | ↑ | Normal, then ↑ | ↑ |
| Respiratory alkalosis | ↑ | ↓ | Normal, then ↓ |
| Table caption - ↑ (up arrow) denotes a rising or increased value, and ↓ (down arrow) denotes a falling or decreased value. | |||
Metabolic acidosis is characterized by lower-than-normal amounts of bicarbonate present in the blood. The PCO₂ is normal at first, but if compensation has occurred, it decreases as the body reestablishes the proper ratio of bicarbonate and carbonic acid/CO₂.
Respiratory acidosis is characterized by excess CO₂ present in the blood. Bicarbonate levels are normal at first, but if compensation has occurred, they increase in an attempt to reestablish the proper ratio of bicarbonate and carbonic acid/CO₂.
Alkalosis is characterized by a higher-than-normal pH. Metabolic alkalosis is characterized by elevated pH and the presence of excess bicarbonate. The PCO₂ is normal at first, but if compensation has occurred, it increases as the body attempts to reestablish the proper ratios of bicarbonate and carbonic acid/CO₂.
Respiratory alkalosis is characterized by CO₂ deficiency in the bloodstream. The bicarbonate concentration is normal at first. When renal compensation occurs, however, the bicarbonate concentration in blood decreases as the kidneys attempt to reestablish the proper ratios of bicarbonate and carbonic acid/CO₂ by eliminating more bicarbonate to bring the pH into the physiological range.
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX "ANATOMY AND PHYSIOLOGY 2E" ACCESS FOR FREE AT OPENSTAX.ORG/DETAILS/BOOKS/ANATOMY-AND-PHYSIOLOGY-2E. LICENSE: CREATIVE COMMONS ATTRIBUTION 4.0 INTERNATIONAL