Table of Contents |
From the moment you wake up in the morning until you go to sleep at night (and through that night as well), you are experiencing chemistry all around you every second, minute, and hour of each day. Most often you do not even notice it, but chemistry is happening all the time. From the elements and compounds in your phone to the fabric in your sheets, to the paint on your wall, to the air you breathe. The respiration that occurs in your body, the food you eat, and everything that happens in and around us each and every day is a result of millions of chemical processes happening right under our noses.
But what is chemistry? We can define chemistry as the study of the composition, properties, and interactions of matter. Matter is anything that occupies space or has mass. But what does that mean?
EXAMPLE
The image below shows a computer, screen, and keyboard, which are all examples of matter.
IN CONTEXT
Throughout human history, people have tried to convert matter into more useful forms. Our Stone Age ancestors chipped pieces of flint into useful tools and carved wood into statues and toys. These endeavors involved changing the shape of a substance without changing the substance itself. But as our knowledge increased, humans began to change the composition of the substances as well—clay was converted into pottery, hides were cured to make garments, copper ores were transformed into copper tools and weapons, and grain was made into bread.
Humans began to practice chemistry when they learned to control fire and used it to cook, make pottery, and smelt metals. Subsequently, they began to separate and use specific components of matter. A variety of drugs such as aloe, myrrh, and opium were isolated from plants. Dyes, such as indigo and Tyrian purple, were extracted from plant and animal matter. Metals were combined to form alloys.
EXAMPLE
Copper and tin were mixed together to make bronze—and more elaborate smelting techniques produced iron. Alkalis were extracted from ashes and combined with fats to make soap. Alcohol was produced by fermentation and purified by distillation.Attempts to understand the behavior of matter extend back for more than 2,500 years. As early as the sixth century BCE, Greek philosophers discussed a system in which water was the basis of all things. You may have heard of the Greek postulate that matter consists of four elements: earth, air, fire, and water.
A combination of chemical technologies and philosophical speculations spread from Egypt, China, and the eastern Mediterranean by alchemists, who endeavored to transform “base metals.” like lead, into “noble metals,” like gold, and create elixirs to cure disease and extend life.
From alchemy came the historical progressions that led to modern chemistry—the isolation of drugs from natural sources, metallurgy, and the dye industry. Today, chemistry continues to deepen our understanding and improve our ability to harness and control the behavior of matter.
This image shows an alchemist’s workshop circa 1580. Although alchemy made some useful contributions to how to manipulate matter, it was not scientific by modern standards. Still, it was a precursor to chemistry.

The image below shows an example of what a chemistry laboratory may look like today.

All of this started by observing our natural world and asking questions. To us, this is just our natural curiosity, but, to chemists, this is the start of true learning and understanding of our natural world. To do this, scientists follow what is called the scientific method.
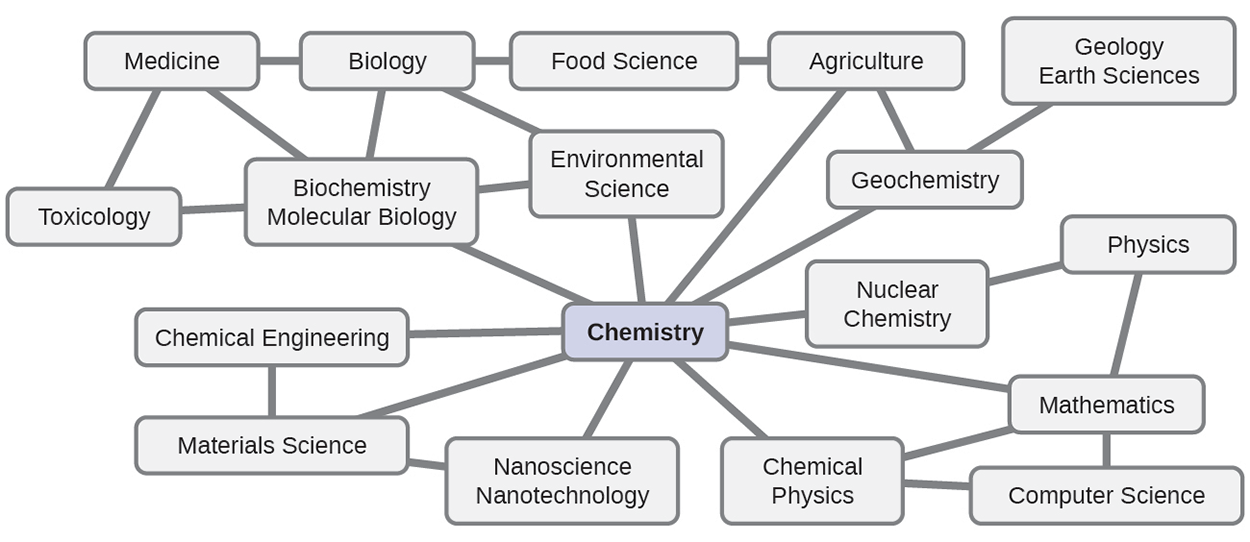
Chemistry is a very complex science that touches on all other fields of science from biology to physics to engineering and many more. Because of this, chemistry is sometimes referred to as “the central science” due to its interconnectedness with a vast array of other STEM disciplines. STEM stands for areas of study in the science, technology, engineering, and math fields. Chemistry and the language of chemists play vital roles in biology, medicine, materials science, forensics, environmental science, and many other fields.
The basic principles of physics are essential for understanding many aspects of chemistry, and there is extensive overlap between many subdisciplines within the two fields.
IN CONTEXT
Chemical physics, nuclear chemistry, mathematics, computer science, and information theory provide important tools that help us calculate, interpret, describe, and generally make sense of the chemical world.
Biology and chemistry converge in biochemistry, which is crucial to understanding the many complex factors and processes that keep living organisms (such as us) alive.
Chemical engineering, materials science, and nanotechnology combine chemical principles findings to produce useful substances, ranging from gasoline to fabrics to electronics.
Agriculture, food science, veterinary science, brewing, and winemaking help provide sustenance in the form of food and drink to the world’s population.
Medicine, pharmacology, biotechnology, and botany identify and produce substances that help keep us healthy.
Environmental science, geology, oceanography, and atmospheric science incorporate many chemical ideas to help us better understand and protect our physical world.
Chemical ideas are used to help understand the universe in astronomy and cosmology.
Knowledge of chemistry is central to understanding a wide range of scientific disciplines. This diagram shows just some of the interrelationships between chemistry and other fields.
EXAMPLE
Digesting and assimilating food, synthesizing polymers (that are used to make clothing, containers, cookware, and credit cards), and refining crude oil (into gasoline and other products) are just a few examples of these changes. Chemistry touches on physics, biology, engineering, and other fields of study all the time.In this course, you will learn about the basic principles of chemistry and the skills needed to understand those principles. You will discover many different examples of changes in the composition and structure of matter. You will learn how to classify these changes, their causes, the changes in energy that accompany them, and the principles and laws involved.
Recall the opening section of this lesson. We defined chemistry as the study of the composition, properties, and interactions of matter. Does that definition make more sense to you now that you see how chemistry interacts all throughout our daily lives, touching upon all the other sciences? In the following lessons and units of this course, you will learn more skills and principles and will be better able to answer the question: “What is chemistry?”
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “CHEMISTRY: ATOMS FIRST 2E”. ACCESS FOR FREE AT Chemistry: Atoms First 2e. LICENSE: CREATIVE COMMONS ATTRIBUTION 4.0 INTERNATIONAL