Table of Contents |
The ecological or biogeochemical cycles, such as the various elements and water cycles, are the biogeochemical processes through which these elements and water are cycled through the ground, air, water and living organisms of the Earth. These are self-sustaining processes to recycle these vital and limited resources that are needed to sustain life on Earth.
These are sometimes called ecological cycles or biogeochemical cycles. Ecological cycles are the various self-regulating processes that recycle the Earth’s limited resources. Biogeochemical cycles are the biological, geological, and chemical processes that occur during each of these cycles.
These elements and water move between both nonliving (abiotic) and living (biotic) components of our ecosystem. Abiotics are non-living chemical and physical parts of the environment that affect living organisms and the functioning of the ecosystem. Biotics are living parts of the environment that affect living organisms and the functioning of the ecosystem. The ecosystem is the biological community of interacting organisms within their physical environment. These elements and water must be continually recycled for living organisms to survive. Each of these elements and water have a reservoir in nature.
We are carbon-based lifeforms. All living organisms are made of carbon and need carbon to survive. The carbon cycle is the way nature recycles carbon through our ecosystem.
EXAMPLE
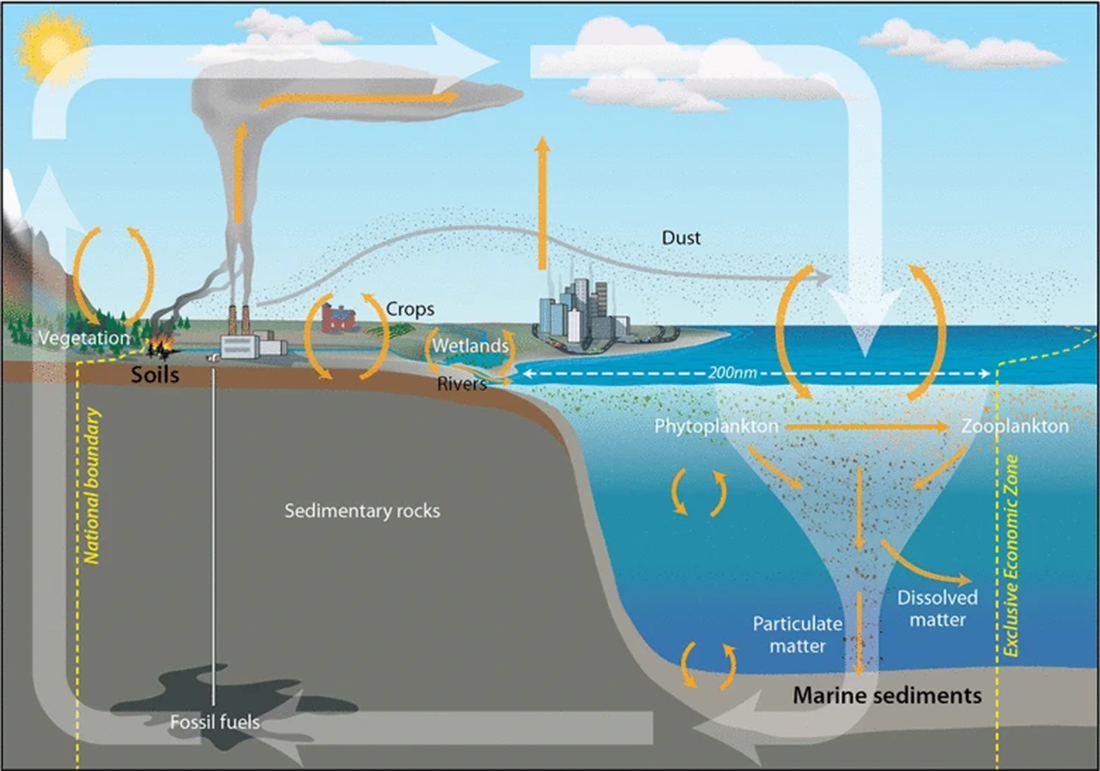
Carbon is found naturally in our atmosphere in the form of carbon dioxide (CO ). It is also found in the minerals and ores of the Earth’s crust and in the ocean and other water sources. Other reservoirs of carbon include all living organisms and decaying plant and animal materials, which we collectively call fossil fuels. Fossil fuels are fuels formed by the natural decaying process of dead organisms from long ago (hence the name fossil). Fossil fuels include oil, coal, natural gas, and peat.
). It is also found in the minerals and ores of the Earth’s crust and in the ocean and other water sources. Other reservoirs of carbon include all living organisms and decaying plant and animal materials, which we collectively call fossil fuels. Fossil fuels are fuels formed by the natural decaying process of dead organisms from long ago (hence the name fossil). Fossil fuels include oil, coal, natural gas, and peat.
In the aquatic portions of the Earth photosynthesis of plankton (tiny organisms that float in the water) and other vegetation takes carbon dioxide as a source of energy out of the water. However, respiration of aquatic animals and decomposition put carbon dioxide back into the water. Additionally, carbon compounds can be absorbed out of the water into the marine sediment or from the sediment into the water source. At the surface, oceans are a huge absorber of excess carbon dioxide from the atmosphere into the water, but at the same time, carbon dioxide is released into the atmosphere from the ocean. All of these processes occur as a steady state with almost equal amounts going into and out of the waterways. Steady state refers to chemical processes that are continually occurring in both directions but have relatively little net change over time.
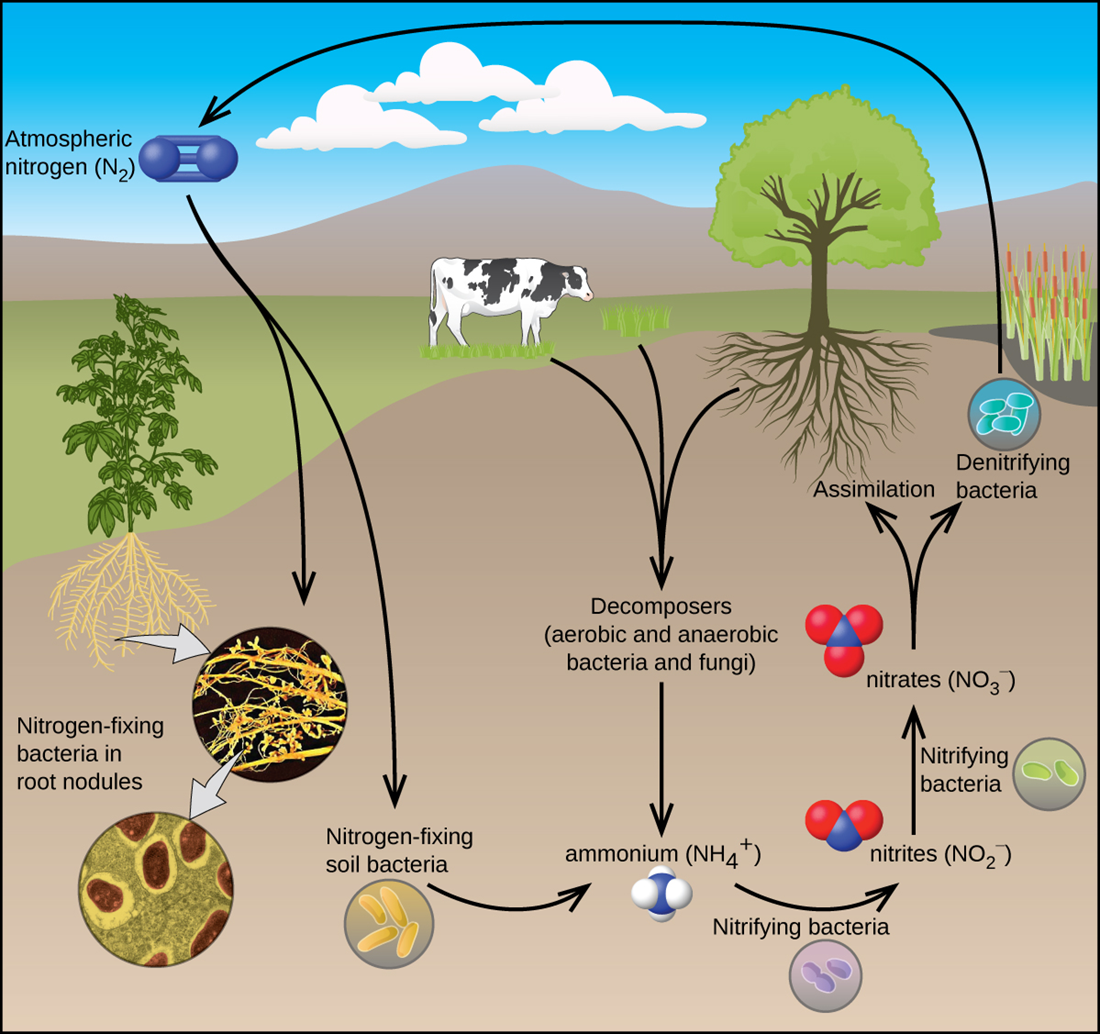
Nitrogen gas (N ) is very unreactive because of the very strong triple bond between the nitrogen atoms. The general lack of reactivity of nitrogen allows for the remarkable ability of some bacteria to synthesize nitrogen compounds using atmospheric nitrogen gas as the source of one of the most exciting chemical events on our planet.
) is very unreactive because of the very strong triple bond between the nitrogen atoms. The general lack of reactivity of nitrogen allows for the remarkable ability of some bacteria to synthesize nitrogen compounds using atmospheric nitrogen gas as the source of one of the most exciting chemical events on our planet.
This process is one type of nitrogen fixation, which is the process where organisms convert atmospheric nitrogen into biologically useful chemicals. Nitrogen fixation also occurs when lightning passes through the air, causing molecular nitrogen to react with oxygen to form nitrogen oxides, which are then carried down to the soil. All living organisms require nitrogen compounds for survival. Unfortunately, most of these organisms cannot absorb nitrogen from their most abundant source—the atmosphere.
The nitrogen cycle is often referred to as nitrogen fixation, as seen in the figure below.
EXAMPLE
To date, the only known kind of biological organisms capable of nitrogen fixation are microorganisms. These organisms employ enzymes called nitrogenases, which contain iron and molybdenum. Many of these microorganisms live in a symbiotic relationship with plants, with the best-known example being the presence of rhizobia in the root nodules of legumes, which are plants that produce their seeds in pods, such as peas and beans .Large volumes of atmospheric nitrogen are necessary for making ammonia—the principal starting material used for preparation of large quantities of other nitrogen-containing compounds. Most other uses for elemental nitrogen depend on its inactivity. It is helpful when a chemical process requires an inert atmosphere.
EXAMPLE
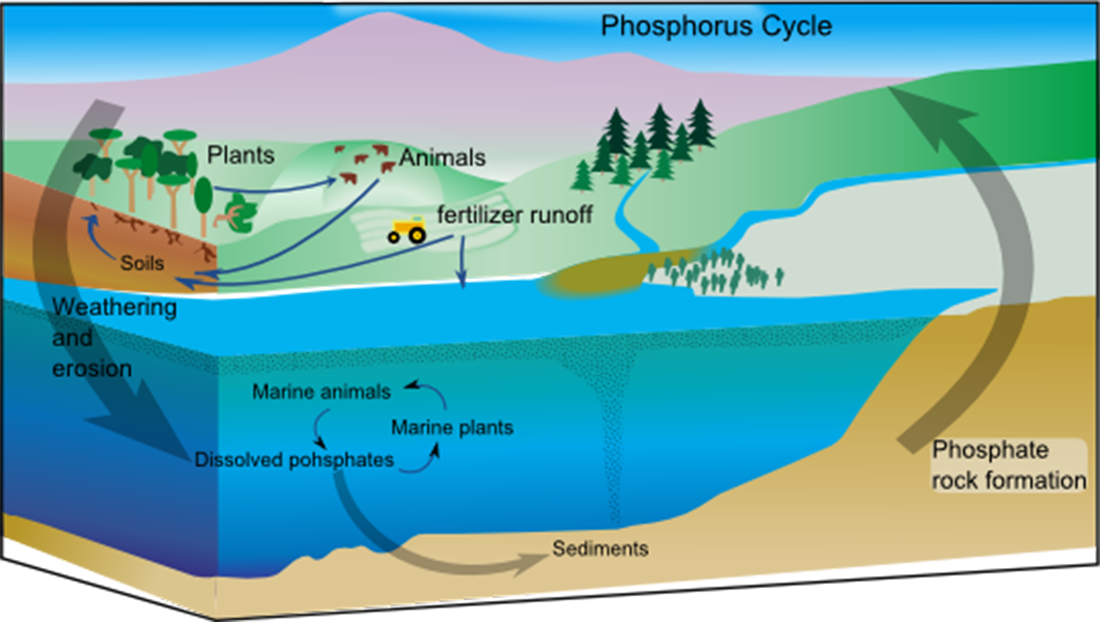
Canned foods and luncheon meats cannot oxidize in a pure nitrogen atmosphere, so they retain a better flavor and color, and spoil less rapidly when sealed in nitrogen instead of air. This technology allows fresh produce to be available year-round, regardless of the growing season.Phosphorus is another important element whose cycle is important for life on Earth. Unlike the other cycles we have talked about so far (and the two remaining cycles), the atmosphere has virtually no role in the cycling of phosphorus. Most phosphorus compounds are solids, aqueous, or bound in living organisms. Most of the phosphorus cycle only uses the solid and water portions of the Earth.
EXAMPLE
Phosphorus is found in our soil and is very important to plant growth. Plants and vegetation take phosphorus out of the soil and fix it in their structures. Humans and animals eat that vegetation and use that as a source of phosphorus for their needs. Decomposition and other processes put that phosphorus back into the land. Many fertilizers and pesticides are rich in phosphorus and runoff from these absorb back into the soil and water supplies.The biggest movement of phosphorus is from surface weathering which brings phosphorus-bearing rocks to the surface from within the Earth’s crust. Also physical erosion—the chemical and biological weathering of these rocks—bring phosphorus compounds into the water supply.
Oxygen is the most abundant element on the Earth and like carbon, nitrogen, and phosphorus is vital to life. All animals breathe oxygen and we cannot live without it. Oxygen plays a major part in all of these cycles. The main chemical in the carbon cycle is carbon dioxide. Nitrites (NO2-) and nitrates (NO3-) are important parts of the nitrogen cycle. Multiple oxygen compounds such as phosphates (PO43-), apatite (Ca5(PO4)₃OH), and other similar minerals and ores are important parts of the phosphorus cycle. Of course, oxygen is involved in the water cycle (oxygen is the O in H2O), as discussed in the next section.
Oxygen and carbon dioxide are exchanged constantly between all animals during respiration (oxygen in and carbon dioxide out as animals breathe). The reverse process occurs in photosynthesis in plants and vegetation (carbon dioxide in and oxygen out during photosynthesis). Another source of oxygen is water, which continually moves from the aquatic (water) to the terrestrial (land) to the atmosphere. This will be talked about in the last section of this lesson.

The carbon, nitrogen, phosphorus, and oxygen cycles were about recycling elements throughout our ecosystem. The last cycle we discuss will be about recycling the compound water throughout our ecosystem. Water is the most abundant compound on Earth. We find it in the water, land, and atmosphere. We also find it in all living organisms.
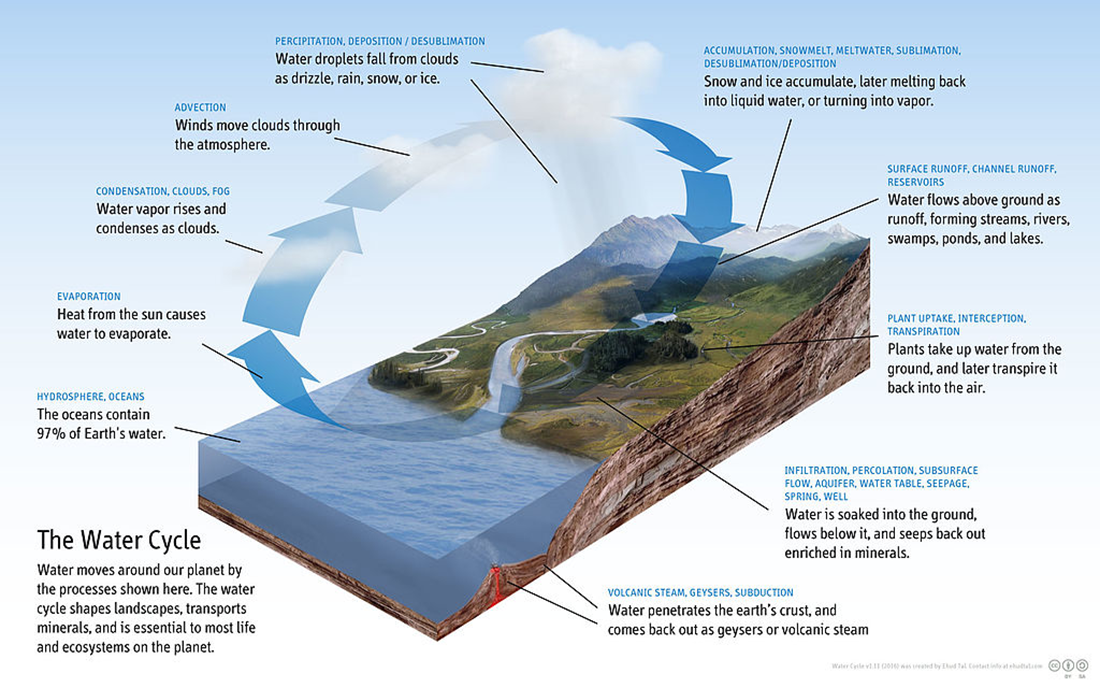
As you can see in the figure below, water moves from the aquatic (lakes, rivers, oceans, seas) portion of the Earth into the atmosphere through evaporation. The water then condenses into the clouds. Water in the form of rain, snow, or ice will fall from the clouds and accumulate in the terrestrial domain (land). From the land, the water will be absorbed into the soil or run off into the aquatic portion of the Earth. The cycle then starts all over.
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “CHEMISTRY: ATOMS FIRST 2E”. ACCESS FOR FREE AT Chemistry: Atoms First 2e. LICENSE: CREATIVE COMMONS ATTRIBUTION 4.0 INTERNATIONAL