Table of Contents |
As you learned in an earlier tutorial, microbes are involved in important biochemical cycles that move elements through ecosystems. Energy enters ecosystems as sunlight (for phototrophs) and as inorganic molecules (for chemotrophs). As you have learned in other tutorials, energy taken up by these autotrophs is used to build organic molecules. The chemicals in these molecules continue to cycle through living organisms and then back into the environment through the activities of living organisms (such as a plants producing oxygen as a waste product) or after the death of an organism.
In addition to their roles in living organisms, the six most common elements associated with organic molecules (carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur) take a variety of chemical forms. They may exist for long periods in the atmosphere, on land, in water, or beneath the Earth’s surface.
EXAMPLE
Carbon is found in biomolecules in living organisms, soil, atmospheric gases, rocks, and minerals.EXAMPLE
Nitrogen is the most abundant gas in the atmosphere, but atmospheric nitrogen (N2) is not usable by most living organisms. Other forms of nitrogen, such as ammonia, are readily available for use by plants and other organisms.Because geological processes (such as erosion, water drainage, movement of continental plates, and weathering) are involved in the cycling of elements on earth, the recycling of inorganic matter between living organisms and their nonliving environment is called biogeochemical cycling.
Examples of major biogeochemical cycles include the carbon cycle, the nitrogen cycle, and the sulfur cycle. In this tutorial, you will learn about these cycles and others with a focus on the role of microbes.
Carbon is a critical component of organic molecules and therefore a critical component of life. However, it is also present in inorganic forms. Organic and inorganic carbon-containing molecules interact in the carbon cycle.
Atmospheric carbon dioxide (CO2), considered inorganic by convention, is essential in the cycling of carbon between organisms. Photoautotrophs like plants take in carbon dioxide and use it to produce organic molecules that are consumed by chemoheterotrophs like animals. Animals oxidize these organic molecules to extract energy and produce carbon skeletons (the carbon chains that form the bases of organic molecules). This process produces carbon dioxide as a waste product.
Carbon dioxide is fully oxidized, but photoautotrophs and chemoautotrophs can use light and chemical energy, respectively, to build reduced organic molecules from carbon dioxide. Heterotrophs then use cellular respiration and fermentation to oxidize these organic molecules to release energy and produce carbon dioxide. As a result, there is a constant exchange of carbon dioxide between heterotrophs that produce carbon dioxide by breaking down organic molecules and autotrophs that use carbon dioxide to build organic molecules. However, it is important to note that autotrophs also break down organic molecules for their own metabolic needs.
EXAMPLE
Soil microbes play an important role in regulating the release or sequestration of carbon. Learning more about these microbes and their activities may be helpful in developing ways to improve crop outputs and increase carbon sequestration, helping to reduce atmospheric levels of carbon dioxide (Gougolias, 2014).EXAMPLE
In some regions, the temperatures are so cold that some ground (permafrost) is frozen throughout the year. As global temperatures increase, some of the permafrost is melting. This means that microbes that have been dormant are becoming active. Permafrost has traditionally been a major carbon sink (meaning that carbon is stored there), but there is considerable concern about increased metabolism and decomposition leading to the increased release of carbon dioxide and methane (a potent greenhouse gas; Brouillette, 2021). Although not directly related to the carbon cycle, this melting also raises concerns that dormant pathogens may emerge and cause new outbreaks of disease or even pandemics (Hofmeister, 2021).The image below summarizes the steps of the sulfur cycle. Atmospheric sulfur can cycle into the ground and then back into the atmosphere. SO2 gas from the atmosphere can be taken up by plants, which use it to produce organic molecules. When animals such as the cows shown eat plants, they produce waste. When they die, they release sulfur-containing compounds as they decompose. Through mineralization, these compounds travel through soil and water as sulfate (SO42−). In a reverse process called immobilization, inorganic sulfur compounds can be converted back to organic forms. Sulfate in soil can become adsorbed or mineral sulfur. It can also form H2S. Lithotrophic bacteria can oxidize H2S to SO. Through bacterial reduction, sulfate in the sediment can be converted to reduced sulfur (H2S, HS−). In the reverse process, bacterial oxidation can convert these back to sulfate. Elemental sulfur (S0) can be oxidized to sulfate.
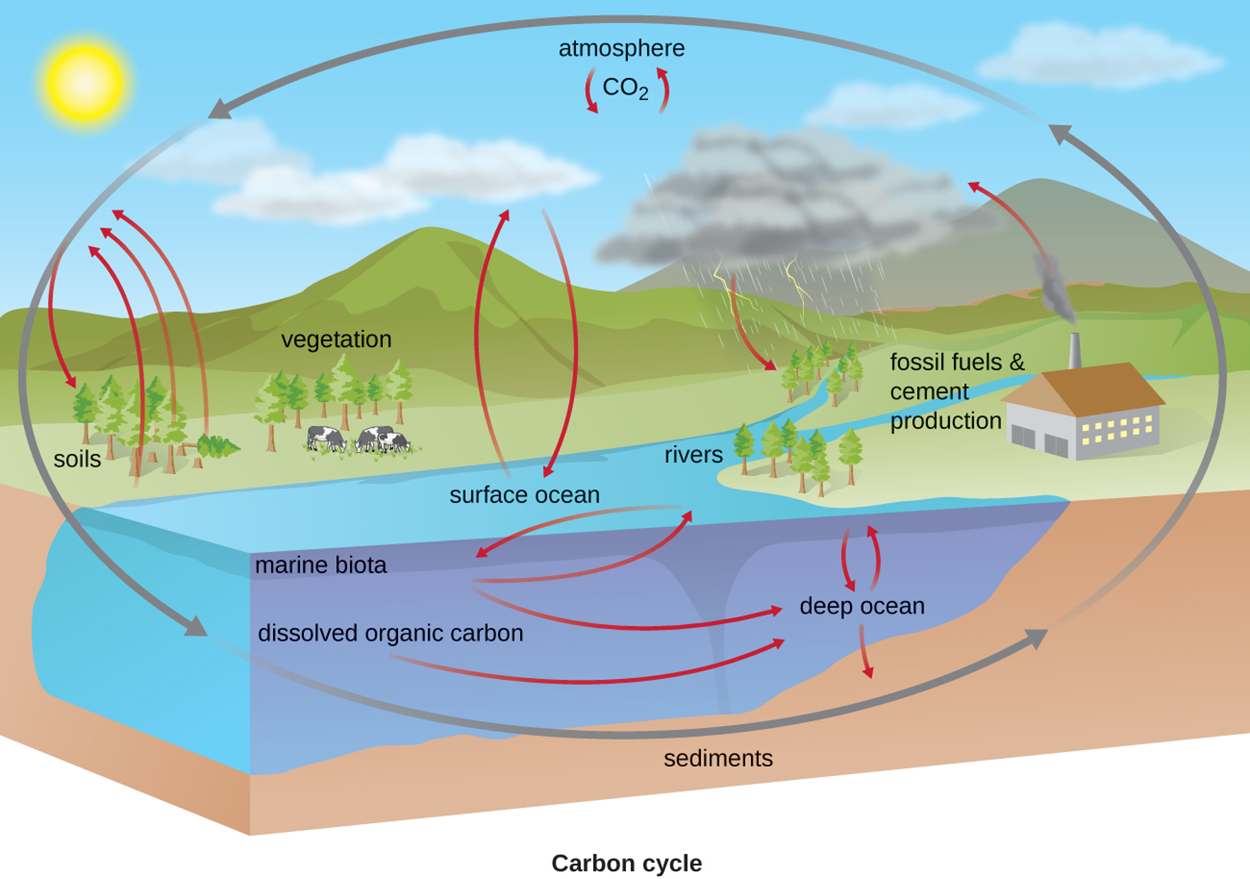
Note that there are many other ways in which carbon interacts with ecosystems. For example, this cycle does not include the roles of methane or of carbon dioxide that forms carbonic acid in water (increasing water acidity).
Thinking about all of the ways in which humans rely on nitrogen makes it clear why the nitrogen cycle is so important. Humans need nitrogen and so do other living organisms that rely on proteins, nucleic acids, and other nitrogenous compounds.
Atmospheric nitrogen (N2) comprises 78% of atmospheric air, but it is not accessible to most organisms. Microbes play an essential role in converting nitrogen to forms that other organisms can use.
Many bacteria are capable of nitrogen fixation, which means that they can incorporate nitrogen into biomolecules. These include several major groups of free-living and symbiotic bacteria.
EXAMPLE
Cyanobacteria in aquatic ecosystems fix inorganic nitrogen from dissolved nitrogen gas (N2) into ammonia (NH3), which is readily available for use by other organisms.EXAMPLE
Some free-living Azotobacter bacteria that can fix nitrogen.EXAMPLE
Rhizobium bacteria form symbiotic associations with legumes such as beans, peanuts, and peas. The bacteria fix nitrogen, providing rich nitrogen resources to the plants. In return, the plants provide fixed carbon as sugars. Rhizobium live in distinctive root nodules.In terrestrial ecosystems, nitrogen gas that undergoes fixation is eventually converted back to nitrogen gas through the following steps.
In aquatic ecosystems, the process is similar except that different microbes (marine bacteria and archaea) perform the necessary reactions.
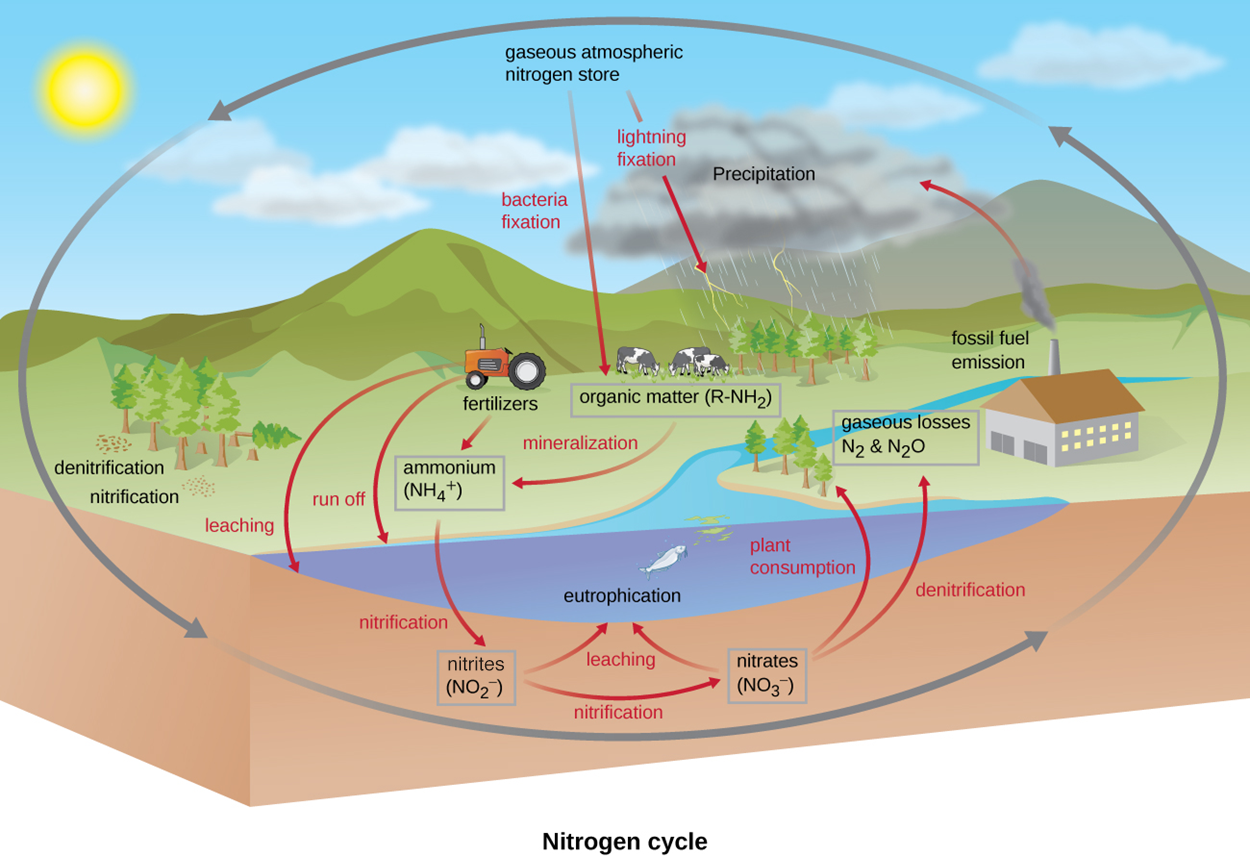
The image below summarizes major steps of the nitrogen cycle and shows how the large reservoir of gaseous atmospheric nitrogen is used throughout the ecosystem. The illustration also shows how overgrowth of microbes due to excess nitrogen can lead to eutrophication.
In the image, the major steps of the nitrogen cycle are shown. There is a large gaseous atmospheric nitrogen store. This can cycle through the air to the ground, through the ground, and back up into the air. Some nitrogen undergoes bacterial fixation or lightning fixation (passing through precipitation) to become available to animals (illustrated as cows) and trees in other forms such as ammonia. These forms of organic matter containing nitrogen are symbolized as R–NH2. Tractors release fertilizers such as ammonium (NH4+). Mineralization can add more nitrogen-rich organic matter to the soil. Runoff and leaching carry nitrogen-rich organic matter from tractors and fields into water, which can lead to eutrophication. Near trees, denitrification and nitrification occur as bacteria take up or convert nitrates and nitrites. An arrow pointing from ammonium from the tractor through the eutrophic lake points to nitrates (NOz−) that can undergo nitrification to produce nitrates (NO3−). Arrows from nitrates and nitrites show that both can enter the lake and contribute to eutrophication. An arrow labeled “plant consumption” points from nitrates to plants on the shore, and an arrow labeled “denitrification” points from nitrates through the water to gaseous losses (N2 and N2O). An arrow labeled “fossil fuel” emission points from smoke leaving the chimney of a house or factory to a cloud labeled “precipitation.”
Sulfur is an important component of organic molecules including proteins and vitamins. For example, the amino acids cysteine and methionine both contain sulfur. Additionally, coenzyme A production requires sulfur.
Multiple groups of microbes use hydrogen sulfide as an electron donor by oxidizing it to elemental sulfur and then to sulfate (SO42−). Hydrogen sulfide is most abundant deeper in the soil where less oxygen is present, where the anoxygenic photosynthetic bacteria and chemoautotrophic archaea and bacteria carry out this process. The sulfate produced can be used as a sulfur source by many bacteria and plants.
Sulfur is readily obtainable by many microbes from sulfate. As dead organisms decompose, sulfur groups are removed from amino acids and hydrogen sulfide is produced. This allows the cycle to continue.
EXAMPLE
Oxidizing of sulfur compounds by microbes is important in wastewater treatment (Wu et al., 2021).The image below summarizes important reactions involving sulfur. Sulfur reduction reactions convert sulfate to hydrogen sulfide in conjunction with heterotrophic sulfate reduction and anaerobic methane oxidation that both produce carbon dioxide, making these reactions relevant to understanding and mitigating global climate change (Cui et al., 2015). In addition to the implications of anaerobic methane oxidation, it is also important to consider the environmental implications of disruptions to the sulfur cycle, which result from acid mine drainage, pollution of waterways, contamination by halogenated persistent organic pollutants (POPs), and increased concentrations of harmful sulfur compounds produced during sewage treatment.
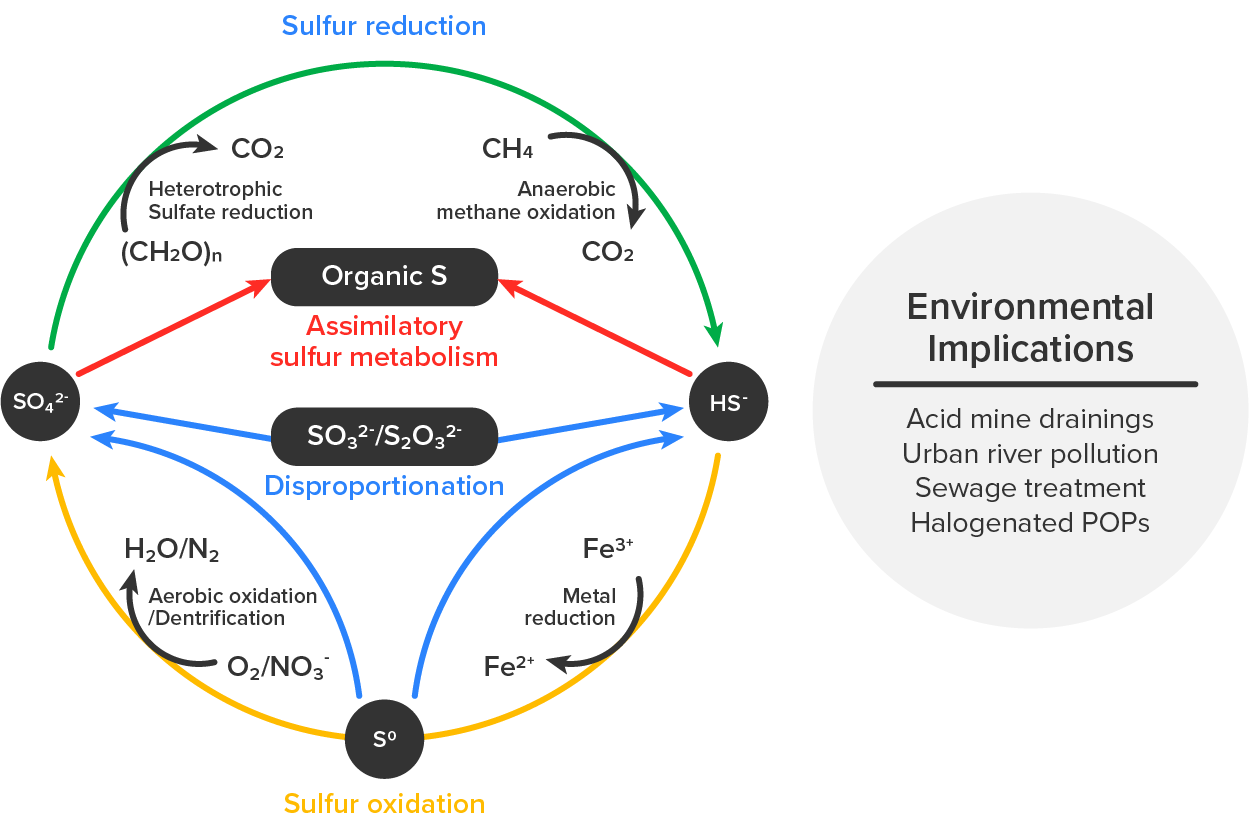
EXAMPLE
Some microbes use sulfur as a terminal electron acceptor for anaerobic electron transport chains. Other microbes reduce sulfur to build organic molecules that can then be used by other microbes (Wu et al., 2021).The image below summarizes the steps of the sulfur cycle. Atmospheric sulfur can cycle into the ground and then back into the atmosphere. SO2 gas from the atmosphere can be taken up by plants, which use it to produce organic molecules. When animals such as the cows shown eat plants, they produce waste. When they die, they release sulfur-containing compounds as they decompose. Through mineralization, these compounds travel through soil and water points as sulfate (SO42−). In a reverse process called immobilization, inorganic sulfur compounds can be converted back to organic forms. Sulfate in soil can become adsorbed or mineral sulfur. It can also form H2S. Lithotrophic bacteria can oxidize H2S to SO. Through bacterial reduction, sulfate in the sediment can be converted to reduced sulfur (H2S, HS−). In the reverse process, bacterial oxidation can convert these back to sulfate. Elemental sulfur (S0) can be oxidized to sulfate.
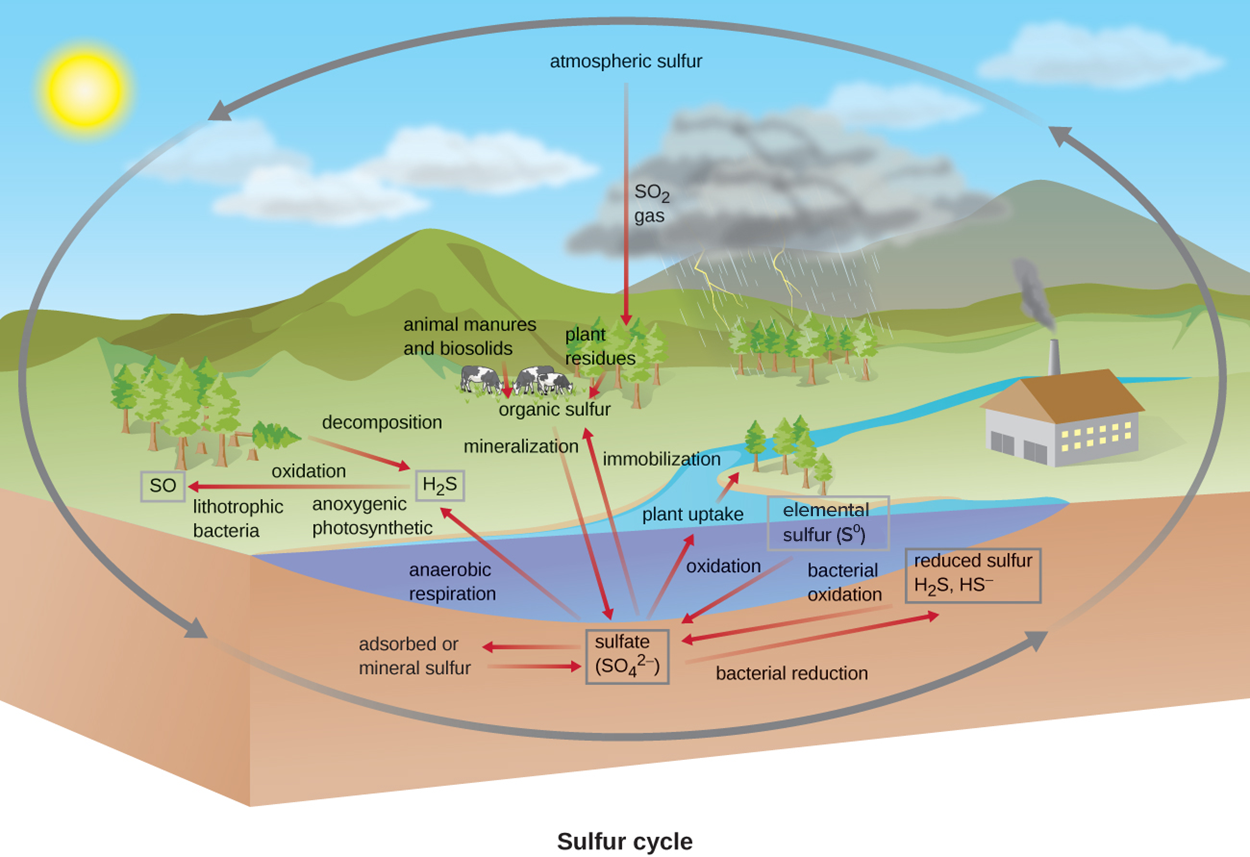
Many other chemicals also participate in important biogeochemical cycles beyond the scope of this tutorial. However, it is important to know that they exist. The cycles can be divided into two types: those that involve oxidation-reduction reactions and those that do not.
The cycles that involve oxidation-reduction reactions include iron, manganese, and chromium, and prokaryotes are important for both oxidation and reduction in each case.
The cycles that do not involve oxidation-reduction reactions include phosphorus, calcium, and silica. These elements are important in the formation of exoskeletons of marine microbes.
Of particular importance are the chemicals that are important in limiting microbial growth: nitrogen, phosphorus, and iron. These chemicals are of particular concern in evaluating the risk of eutrophication. Nitrogen has been discussed previously because of its important role in fertilizer, although it has additional important roles. Phosphorus is commonly used in detergents and therefore is also a significant concern.
Because microbes are capable of such varied chemical reactions, many of which are not possible by animals and plants, they have valuable capabilities for bioremediation. Bioremediation means using biological means to “remediate” (i.e., clean up) a location that has become contaminated by pollutants.
In discussions of bioremediation, the term xenobiotics is often used because “xeno” refers to a foreign or unnatural substance (Merriam-Webster, N.d.). Pollutants or substances artificially introduced at unusually high concentrations can both be considered xenobiotics. Examples include adhesives, dyes, flame retardants, lubricants, oil and petroleum products, organic solvents, pesticides, and products of the combustion of gasoline and oil.
Because xenobiotics are not natural, they are often difficult to break down and may accumulate in the food chain. They harm wildlife and may also harm humans, both directly (through consumption) and indirectly (through economic impacts such as loss of tourism opportunities or fisheries).
Bioremediation is one way to deal with contaminants and can be conducted in two ways. In situ bioremediation occurs when treatment takes place at the contaminated site and no material is moved. Ex situ bioremediation involves moving material elsewhere for treatment.
Additionally, bioremediation approaches can be enhanced in various ways. For example, some bioremediation processes rely on microorganisms that are naturally present at the contaminated site or in the material. Others involve the addition of non-native microbes that are especially effective at degrading the contaminant or in helping to make the contaminant more amenable to removal.
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “MICROBIOLOGY.” ACCESS FOR FREE AT openstax.org/details/books/microbiology. LICENSE: CC ATTRIBUTION 4.0 INTERNATIONAL.
REFERENCES
Brouillette M. (2021). How microbes in permafrost could trigger a massive carbon bomb. Nature, 591(7850), 360–362. doi.org/10.1038/d41586-021-00659-y
Cui, M., Ma, A., Qi, H., Zhuang, X., & Zhuang, G. (2015). Anaerobic oxidation of methane: an "active" microbial process. MicrobiologyOpen, 4(1), 1–11. doi.org/10.1002/mbo3.232
Gillezeau, C., van Gerwen, M., Shaffer, R. M., Rana, I., Zhang, L., Sheppard, L., & Taioli, E. (2019). The evidence of human exposure to glyphosate: a review. Environmental health: a global access science source, 18(1), 2. ehjournal.biomedcentral.com/articles/10.1186/s12940-018-0435-5
Gougoulias, C., Clark, J. M., & Shaw, L. J. (2014). The role of soil microbes in the global carbon cycle: tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. Journal of the science of food and agriculture, 94(12), 2362–2371. doi.org/10.1002/jsfa.6577
Hofmeister, A. M., Seckler, J. M., & Criss, G. M. (2021). Possible Roles of Permafrost Melting, Atmospheric Transport, and Solar Irradiance in the Development of Major Coronavirus and Influenza Pandemics. International journal of environmental research and public health, 18(6), 3055. doi.org/10.3390/ijerph18063055
Merriam-Webster. (n.d.). Xenobiotic. In Merriam-Webster.com dictionary. Retrieved September 23, 2022, from www.merriam-webster.com/dictionary/xenobiotic
Miura, R., Araki, A., Minatoya, M., Miyake, K., Chen, M. L., Kobayashi, S., Miyashita, C., Yamamoto, J., Matsumura, T., Ishizuka, M., Kubota, T., & Kishi, R. (2019). An epigenome-wide analysis of cord blood DNA methylation reveals sex-specific effect of exposure to bisphenol A. Scientific reports, 9(1), 12369. doi.org/10.1038/s41598-019-48916-5
Morgado, M., Ascenso, C., Carmo, J., Mendes, J. J., & Manso, A. C. (2022). pH analysis of still and carbonated bottled water: Potential influence on dental erosion. Clinical and experimental dental research, 8(2), 552–560. doi.org/10.1002/cre2.535
Ruiz, S. A., McKay Fletcher, D. M., Boghi, A., Williams, K. A., Duncan, S. J., Scotson, C. P., Petroselli, C., Dias, T., Chadwick, D. R., Jones, D. L., & Roose, T. (2020). Image-based quantification of soil microbial dead zones induced by nitrogen fertilization. The Science of the total environment, 727, 138197. doi.org/10.1016/j.scitotenv.2020.138197
Wu, B., Liu, F., Fang, W., Yang, T., Chen, G. H., He, Z., & Wang, S. (2021). Microbial sulfur metabolism and environmental implications. The Science of the total environment, 778, 146085. doi.org/10.1016/j.scitotenv.2021.146085