Table of Contents |
In other lessons, you have learned about the difference between horizontal gene transfer (HGT) and vertical gene transfer. Unlike vertical gene transfer, HGT transfers genetic material between organisms that are not parent and offspring.
Unlike vertical gene transfer, HGT can occur between organisms of different or even distantly related species. As researchers have become increasingly aware of how common HGT is, they have found more and more examples.
The image below shows the three major mechanisms of HGT in bacteria. These mechanisms have been well studied in bacteria. They are believed to occur in some archaeans, but less is known about these and other potential methods of HGT in archaea (Wagner et al., 2017). Therefore, this lesson will discuss each of these mechanisms in bacteria in more detail below.
The figure below summarizes the mechanisms in the following three parts:
Part (a) Transformation: A bacterial cell takes up naked DNA from the environment.
Part (b) Transduction: A bacteriophage transfers genes from one bacterium to another.
Part (c) Conjugation: One bacterium (the donor) extends a hollow tube (a conjugation pilus or sex pilus) to another bacterium (the recipient) and genetic material moves through the pilus from the donor to the recipient.
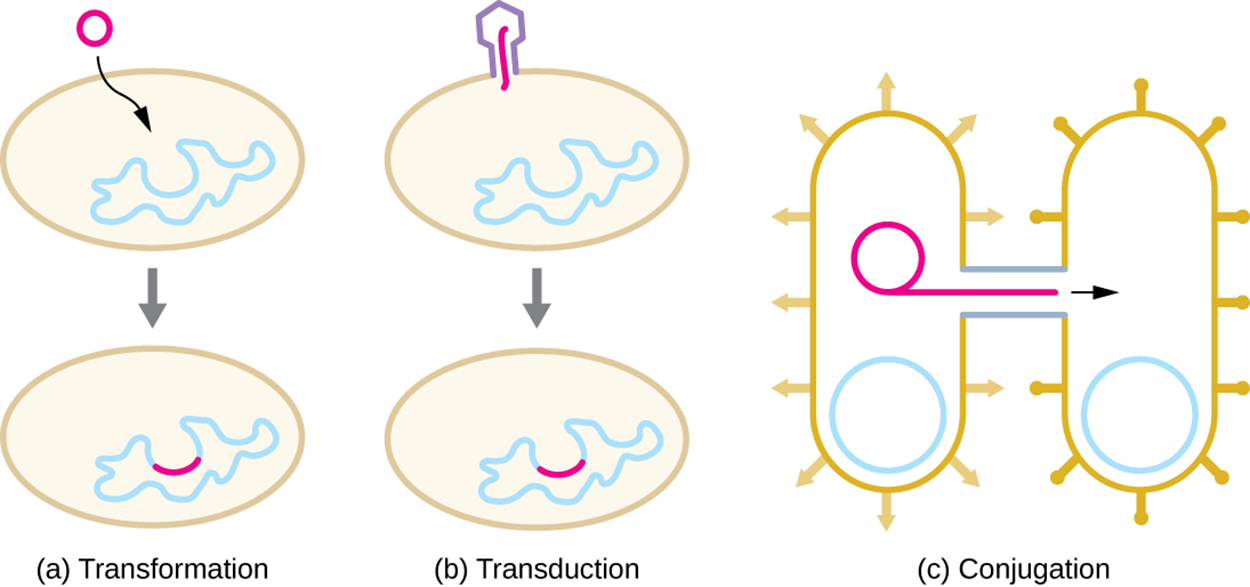
In transformation, a bacterial cell encounters naked DNA in the environment, takes up the DNA, and is able to take up genes from the DNA through recombination. Bacteria that are capable of this are called competent and this ability can occur naturally or be induced in a laboratory setting.
When unfamiliar double-stranded DNA enters cells, it is generally destroyed by enzymes called nucleases. This helps to protect against viral infection. Therefore, competent cells that transport DNA across their membranes must also make the DNA single stranded to protect it from degradation. Transformation can also be used to take up plasmids from the environment.
Once this single-stranded DNA is in the cell, it can recombine with the bacterial genome. In other words, it can exchange genetic material with existing DNA. This can alter the phenotype of the bacterium.
Although transformation is important, especially for the acquisition of genes associated with virulence factors and antibiotic resistance, it is inefficient in nature. Naked DNA (meaning DNA that is loose in the environment rather than enclosed in a cell or viral capsid) can be left in the environment when cells die. However, environmental DNA suitable for transformation is not abundant because nucleases often degrade this DNA. Additionally, genetic recombination is inefficient at incorporating new DNA sequences into the genome.
You will learn more about ways in which transformation is used in the laboratory in the lessons on biotechnology.
Bacteriophages (viruses that infect bacteria) can sometimes transfer small pieces of DNA from one bacterium to another. This process is called transduction.
In the lesson on virus life cycles, you learned that viral genetic material is incorporated into the host chromosome as a prophage during the lysogenic life cycle of bacteriophages. When the prophage is excised from the chromosome, it may carry a piece of adjacent bacterial chromosome with it. When new phages are produced, this small piece of bacterial chromosome may be packaged into them. This means that the phage injects the bacterial chromosome as well as viral genes when it infects a new host cell.
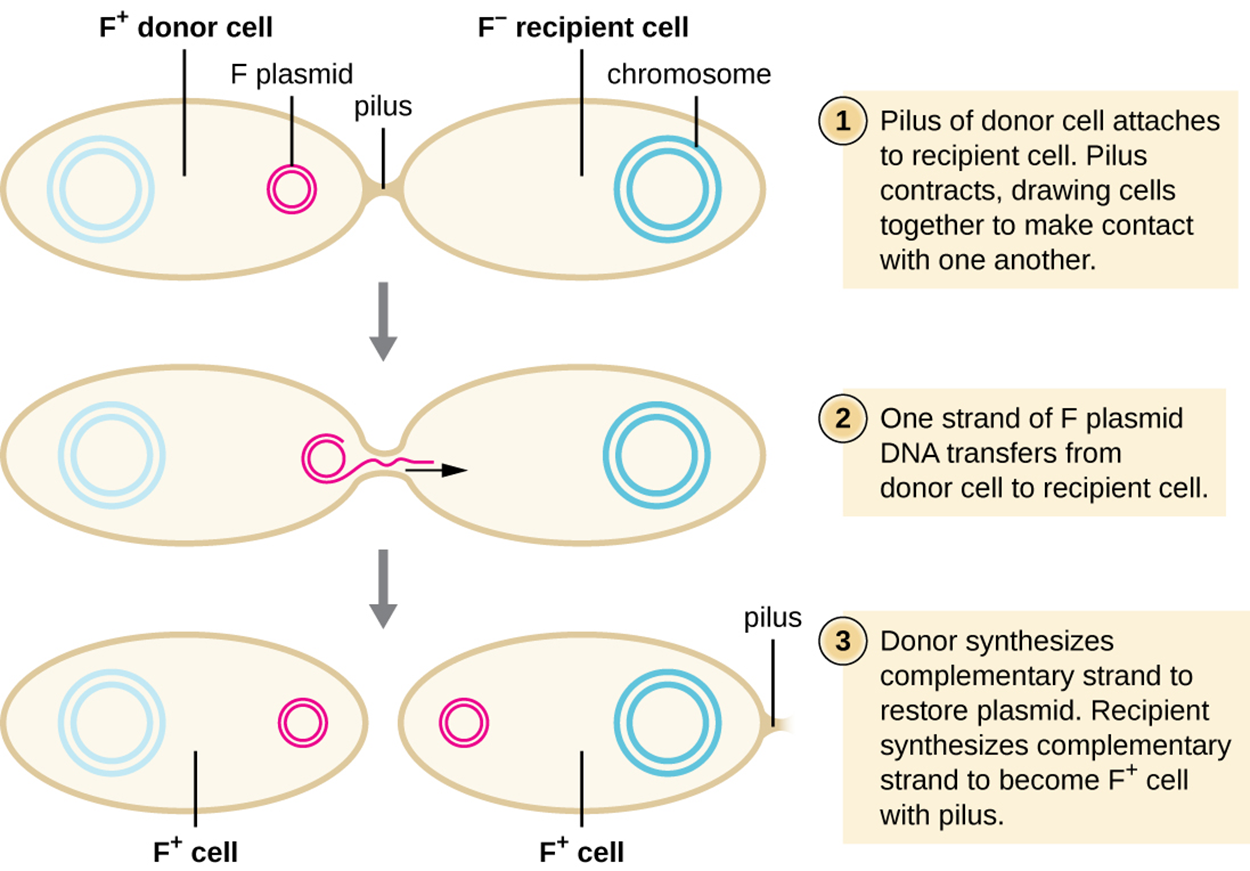
When DNA is transferred from one bacterium to another through a conjugation pilus, the process is called conjugation.
In Escherichia coli, the genes responsible for conjugation are located on a bacterial plasmid called the F plasmid (fertility factor) and the conjugation pilus is called the F pilus. Bacterial cells that contain the fertility factor are called F+ or donor cells because they contribute DNA to other cells. Cells that lack the fertility factor are called F− or recipient cells because they receive DNA.
The F plasmid contains genes that encode the F pilus as well as genes for rolling circle replication of the plasmid. During conjugation, the plasmid replicates using rolling circle replication with one plasmid remaining in the donor cell while the displaced strand produced through rolling circle replication moves through the pilus to the recipient cell.
The steps and image below illustrate the process of conjugation in E. coli.
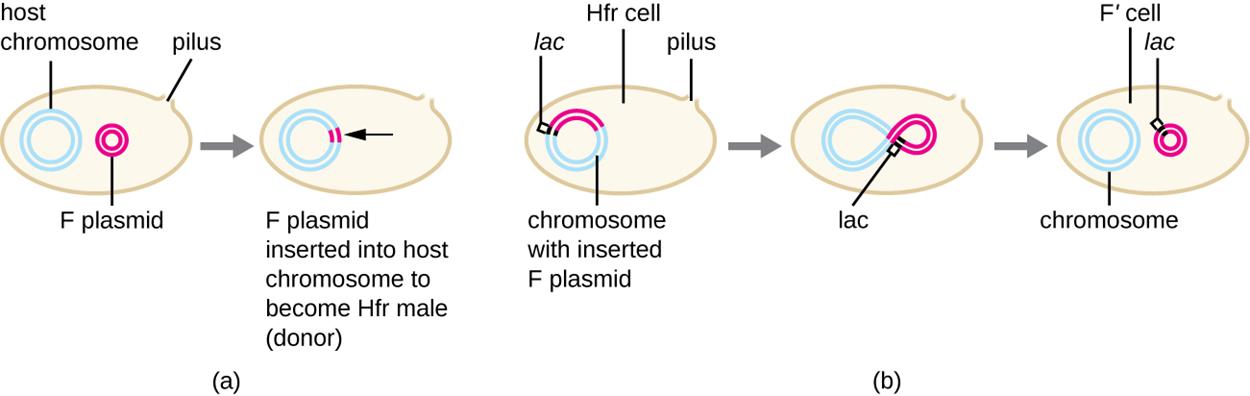
Conjugation can sometimes transfer chromosomal DNA in addition to the F plasmid. When conjugation occurs as described above, only the F plasmid is transferred. However, as shown in part (a) of the image below, the F plasmid can be integrated into the main host chromosome. When this happens, the cell is called an Hfr cell (Hfr refers to high frequency of recombination).
Part (b) of the image shows one possible outcome when an Hfr cell is formed. If the plasmid is excised from the chromosome, small pieces of chromosomal DNA may be included in the plasmid. When this happens, the plasmid is called an F′ plasmid. When an F′ plasmid undergoes conjugation, it transfers chromosomal DNA. For example, part (b) of the image shows that a gene labeled “lac” is transferred as part of the F′ plasmid.
Another possible outcome of Hfr formation is that conjugation can occur while the F plasmid is still integrated into the main chromosome as shown in part (a) of the image below. When this happens, part of the chromosome is transferred. However, the chromosome is so large that conjugation is almost always interrupted before the entire chromosome is transferred and therefore the recipient cell rarely receives a full F plasmid.
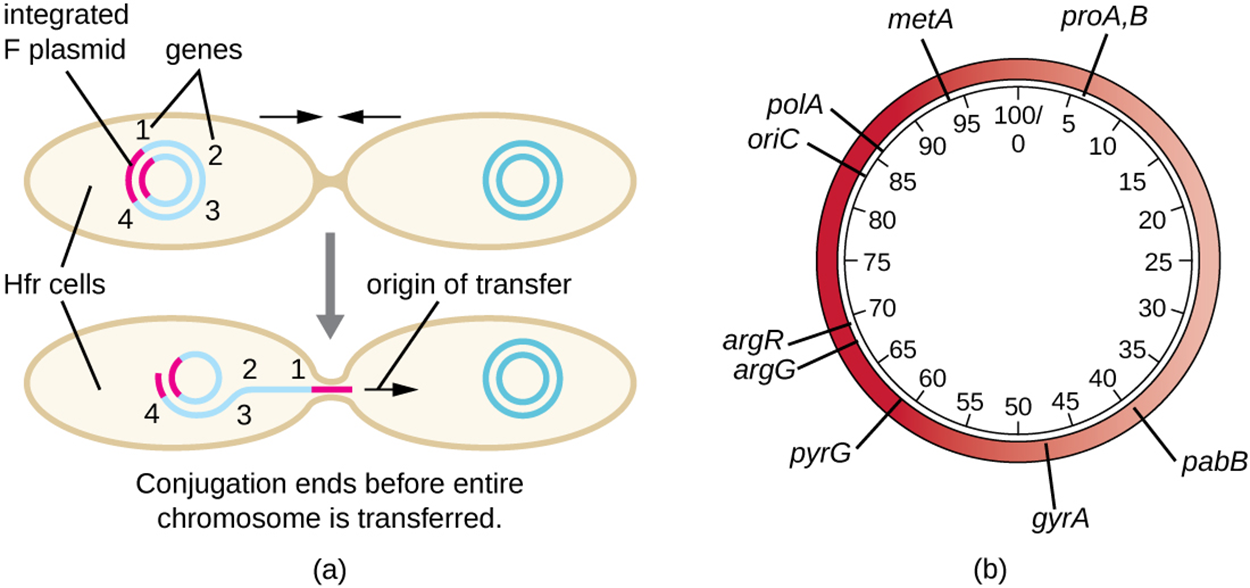
Because genes that are close together on the chromosome are more likely to be transferred together, mapping could be accomplished by interrupting conjugation at different times. In part (a) of the image above, there are genes labeled 1 through 4. A short period of conjugation might allow only gene 1 to transfer. A slightly longer period of conjugation might allow genes 1 and 2 to transfer. Even longer periods could allow 3 to transfer as well, or even 4. In this way, a map could be produced with distances measured in minutes as shown in part (b) of the image.
This type of mapping is time consuming and no longer needed as newer sequencing techniques are so readily available. You will learn more about other approaches to mapping and sequencing in other lessons.
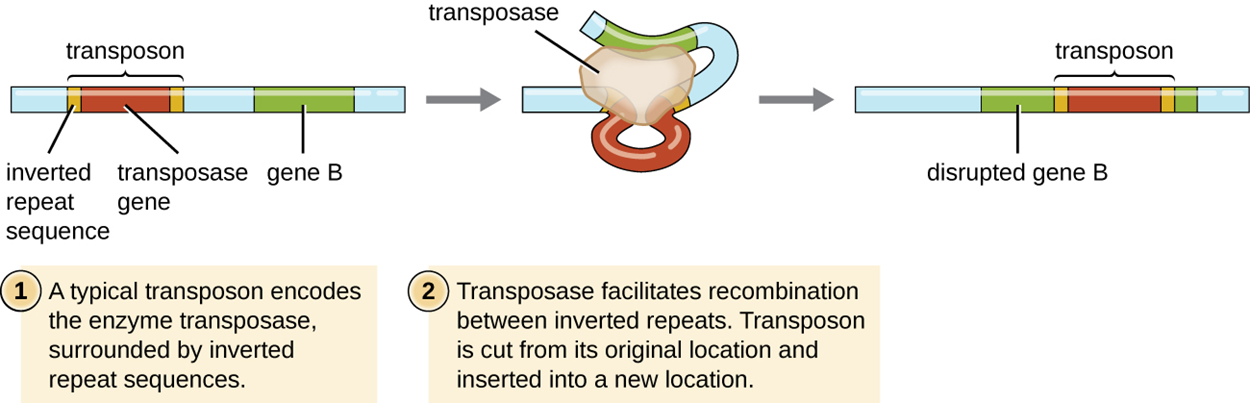
Another way in which asexual organisms can generate genetic diversity is through transposition, which occurs in a wide variety of organisms beyond asexual microbes. In fact, transposition was first discovered in maize (corn).
In transposition, genetic elements called transposons (transposable elements) or “jumping genes” can excise themselves from one location in DNA and integrate into the same or a different molecule elsewhere. Transposons have special inverted repeats at their ends. They also have a gene for the enzyme transposase.
Although some transposons replicate and leave a copy at their original location while the new copy is inserted elsewhere, most do not replicate and simply move from one location to another.
Transposons can transport additional genes such as antibiotic resistance genes and therefore give a new function to a cell. They can also disrupt existing genes, causing them to cease to function.
The steps and image below illustrate the steps of transposition for a transposon that does not replicate itself.
The table below summarizes mechanisms that promote genetic diversity in prokaryotes. In future lessons, you will learn more about the importance of these mechanisms and how some are used in research and clinical settings. Note that transposition also occurs in eukaryotes.
| Summary of Mechanisms of Genetic Diversity in Prokaryotes | |
|---|---|
| Term | Definition |
| Conjugation | Transfer of DNA through direct contact using a conjugation pilus |
| Transduction | Mechanism of HGT in bacteria in which genes are transferred through viral infection |
| Transformation | Mechanism of HGT in which naked environmental DNA is taken up by a bacterial cell |
| Transposition | Process whereby DNA independently excises from one location in a DNA molecule and integrates elsewhere |
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “MICROBIOLOGY.” ACCESS FOR FREE AT openstax.org/details/books/microbiology. LICENSE: CC ATTRIBUTION 4.0 INTERNATIONAL.
REFERENCES
Wagner, A., Whitaker, R. J., Krause, D. J., Heilers, J. H., van Wolferen, M., van der Does, C., & Albers, S. V. (2017). Mechanisms of gene flow in archaea. Nature reviews. Microbiology, 15(8), 492–501. doi.org/10.1038/nrmicro.2017.41