Table of Contents |
As you learned in earlier lessons, antibodies (immunoglobulins) have variable regions that allow different antibodies to bind very selectively to particular epitopes of an antigen. The image below shows an example of an antigen with three epitopes, each of which is bound by a different antibody.
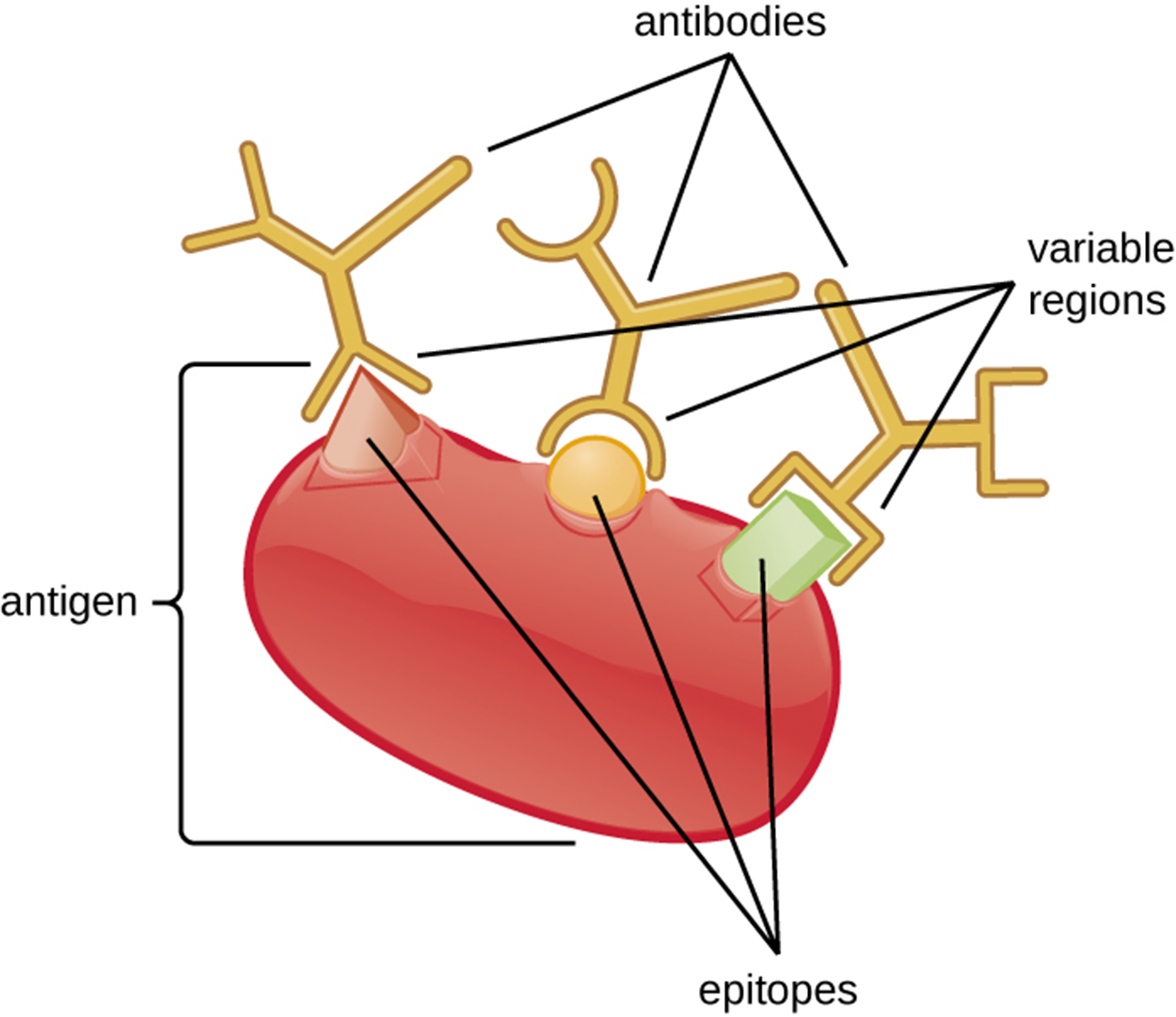
Antibodies have specificity, which means that each antibody can only bind to the specific epitope targeted by the antigen-binding site of its variable region. This does not mean that each antibody can only bind to a single epitope. Because some epitopes are sufficiently chemically similar, the variable region of the antibody can bind to more than one epitope. This phenomenon of an antibody binding to more than one similar antigen is called cross-reactivity. An individual antigen can have multiple epitopes and therefore may be targeted by multiple antibodies. This can increase the probability of cross-reactivity if some antigens share similar epitopes.
Antibodies can bind to antigens with different amounts of strength. The measure of the binding strength between an antibody’s binding site and an epitope is determined experimentally and called affinity. In contrast, avidity is the total strength of all interactions in an antibody–antigen complex, including binding between antibodies and multiple epitopes, if present. Affinity and avidity interact.
Knowing about affinity and avidity is helpful in predicting the likelihood of cross-reactivity. Cross-reactivity is less likely when the avidity and affinity of an antibody are high for a particular antigen. Cross-reactivity is more likely when the avidity and affinity of the antibody are low for an antigen.
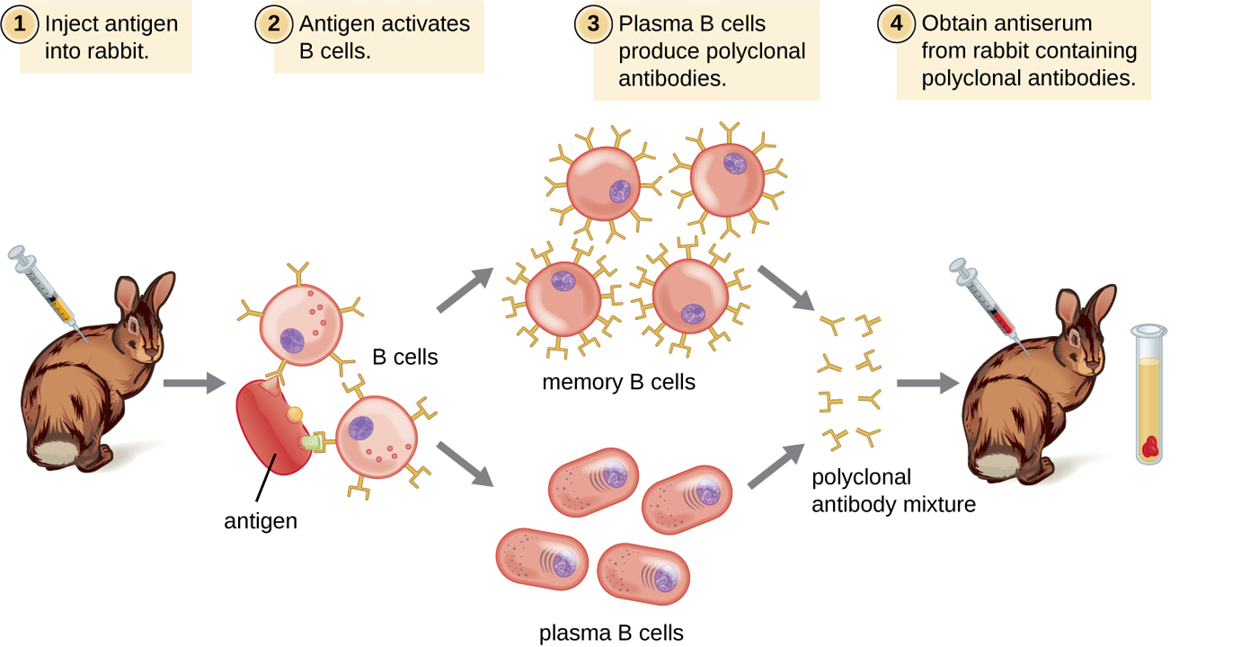
When antibodies are needed for laboratory purposes such as research, they are often produced by injecting an antigen into an animal such as a rabbit or a goat. After a sufficient amount of time (a few weeks), the animal’s immune system produces large numbers of antibodies specific to the antigen. These antibodies are present in whole serum that can be taken from the animal. When serum contains antibodies for a particular antigen, it is called antiserum (plural = antisera). Because antiserum contains a range of antibodies depending on which antigens an animal has previously been exposed to, it is purified to isolate the antibodies of interest for a particular purpose such as research.
Antibodies are often complex structures with multiple epitopes, meaning that a variety of antibodies is generally produced. This is called a polyclonal antibody response because multiple different clones of B cells (each responding to a different epitope of the antigen) are produced. These combinations of clones are called polyclonal antibodies.
To make polyclonal antibodies, animals are usually injected with antigen at least twice to produce antiserum to be harvested. The second injection activates memory cells that make class immunoglobulin G (IgG) antibodies against the antigen. The memory cells also undergo affinity maturation, resulting in a pool of antibodies with higher average affinity.
Affinity maturation occurs because of mutations in the immunoglobulin gene variable regions that produce B cells with slightly altered antigen-binding sites. After the second exposure to the antigen, B cells that produce antibody with higher affinity antigen-binding sites will be stimulated to proliferate and produce more antibody than B cells that produce lower-affinity antibodies.
In many cases, an adjuvant (a chemical that stimulates the immune system to increase antibody production) is mixed with the antigen prior to injection. Adjuvants are also used in vaccines for the same reason (see Saya et al., 2022).
The steps of polyclonal antibody production are summarized in the steps and image below.
Polyclonal antisera are often used in clinical tests to determine whether a patient is producing antibodies to a particular pathogen (suggesting infection by that pathogen). They can also be used to activate complement, detect the presence of bacteria in clinical and food industry settings, and perform a wide array of reactions that can detect and quantify proteins or other antigens. You will learn more about some of these reactions in other lessons.
However, an important consideration in using polyclonal antisera is that they often exhibit cross-reactivity. This increases the risk of a false-positive result, which is a positive test result when the result should be negative (in this case, a test that shows the presence of an antigen that is actually not present). False-negative results can also occur in diagnostic testing, including testing using polyclonal antisera. A false-negative result is a negative result when the test should be positive (in this case, when a test indicates the absence of an antigen that is actually present).
The accuracy of antibody (and other) tests can be described in terms of test sensitivity and test specificity. Test sensitivity is the probability of obtaining a positive test result that is accurate (in this case, the antigen really is present). Test specificity is the probability of obtaining an accurate negative test result (in this case, when the antigen is actually absent).
Another limitation of testing using polyclonal antisera is that antibodies may be present long after a pathogen has been eliminated by the immune system. Therefore, this type of testing can indicate that someone has experienced an infection but does not determine whether they have a current infection.
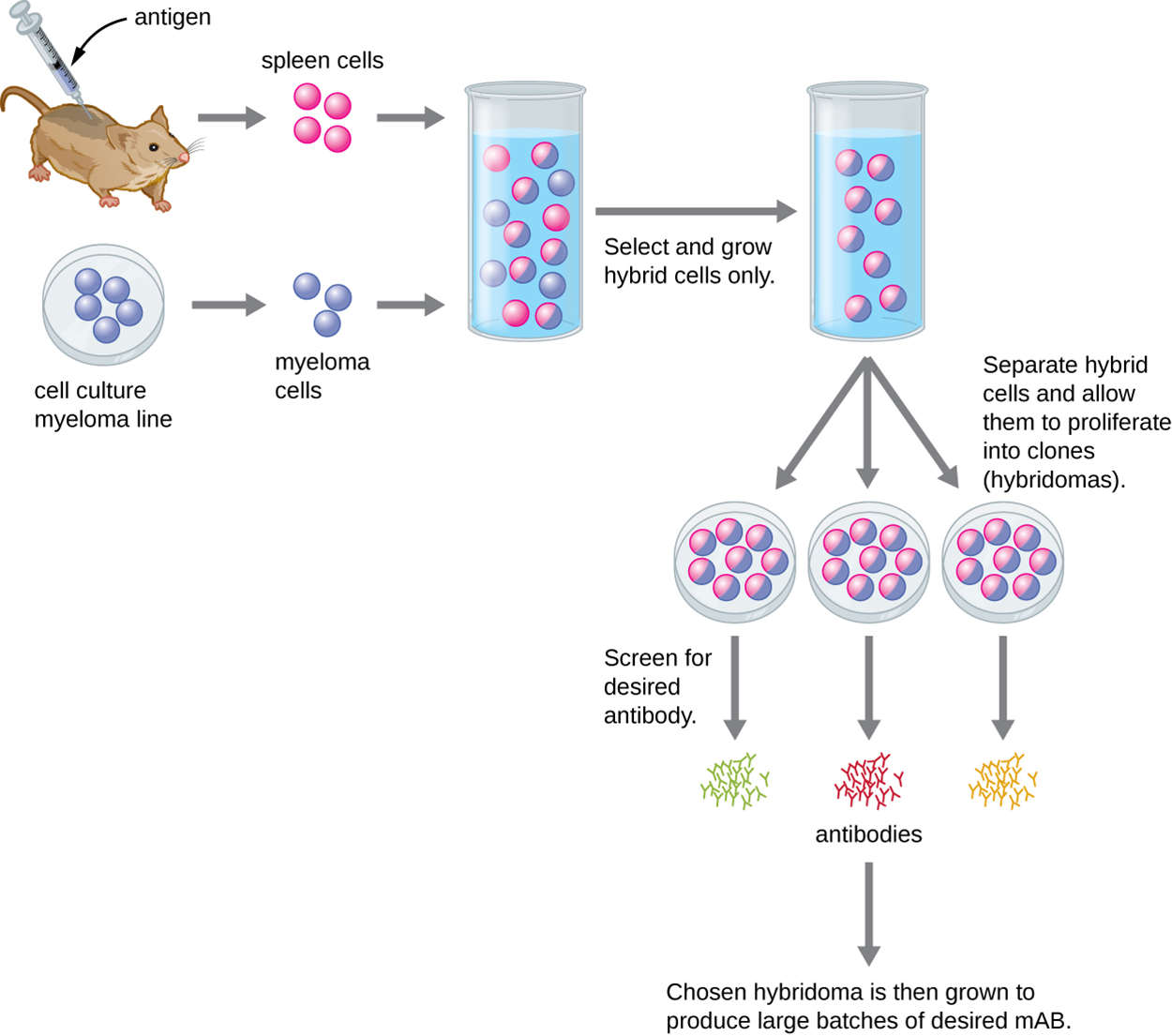
Monoclonal antibodies (mAbs) target single epitopes and are used for assays that require higher antibody specificity and affinity than can be obtained using polyclonal antisera. These antibodies are produced in vitro using tissue culture techniques rather than in live animals. The process, which is described in the steps and image below, is more expensive and time consuming than the production of polyclonal antibodies.
The table below compares major characteristics of polyclonal and monoclonal antibodies.
| Characteristics of Polyclonal and Monoclonal Antibodies | |
|---|---|
| Monoclonal Antibodies | Polyclonal Antibodies |
| Expensive production | Inexpensive production |
| Long production time | Rapid production |
| Large quantities of specific antibodies | Large quantities of nonspecific antibodies |
| Recognize a single epitope on an antigen | Recognize multiple epitopes on an antigen |
| Production is continuous and uniform once the hybridoma is made | Different batches vary in antibody composition |
Because most monoclonal antibodies are produced in mouse cells, it is necessary to create humanized monoclonal antibodies for clinical use. Otherwise, the human immune system will recognize them as foreign and will respond by producing neutralizing antibodies.
Humanized monoclonal antibodies are produced by genetically engineering the antibody in the mouse B cell. The variable regions of the mouse light and heavy genes are ligated to human constant regions and the products of this process are transferred into a host cell. The host cell produces an mAb that is mostly human but has the unique antigen-binding site produced by the mouse cell.
Humanized monoclonal antibodies have been used to treat cancer and are in development for the treatment of infectious diseases. mAbs have been used to treat COVID-19 in certain patients, although some resistance has been reported in emerging viral variants (Cox et al., 2022; Yetmar et al., 2022).
There is also interest in using genetically engineered plants to produce antibodies, called plantibodies. Genes inserted into the plant cells cause the plant cells to produce the required antibodies. It is possible that these antibodies could be used by having patients consume plants, which would eliminate steps that slow down production of mAbs.
Source: THIS TUTORIAL HAS BEEN ADAPTED FROM OPENSTAX “MICROBIOLOGY.” ACCESS FOR FREE AT openstax.org/details/books/microbiology. LICENSE: CC ATTRIBUTION 4.0 INTERNATIONAL.
REFERENCES
Cox, M., Peacock, T. P., Harvey, W. T., Hughes, J., Wright, D. W., COVID-19 Genomics UK (COG-UK) Consortium, Willett, B. J., Thomson, E., Gupta, R. K., Peacock, S. J., Robertson, D. L., & Carabelli, A. M. (2022). SARS-CoV-2 variant evasion of monoclonal antibodies based on in vitro studies. Nature reviews. Microbiology, 1–13. Advance online publication. doi.org/10.1038/s41579-022-00809-7
Seya, T., Tatematsu, M., & Matsumoto, M. (2022). Toward Establishing an Ideal Adjuvant for Non-Inflammatory Immune Enhancement. Cells, 11(24), 4006. doi.org/10.3390/cells11244006
Yetmar, Z. A., Bhaimia, E., & Razonable, R. R. (2022). Antispike monoclonal antibodies for prevention and treatment of coronavirus disease-2019 in solid organ transplant recipients. Current opinion in organ transplantation, 27(4), 269–276. doi.org/10.1097/MOT.0000000000000981