Table of Contents |
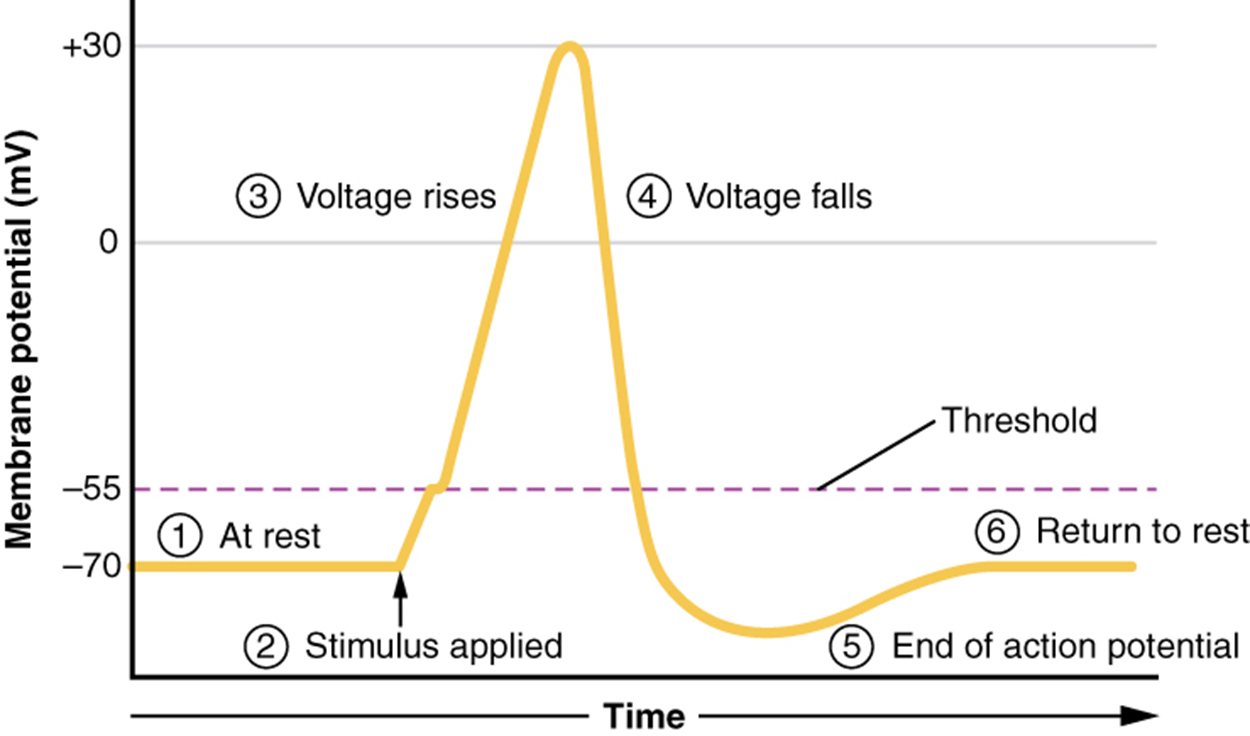
Resting membrane potential describes the steady state of the cell, which is a dynamic process balanced by ion leakage and ion pumping. Without any outside influence, it will not change. However, an action potential is a change in the membrane potential of an excitable cell in response to a stimulus. This stimulus disturbs the membrane potential and causes the creation of an action potential. The first step of an action potential is the opening of voltage-gated sodium channels.
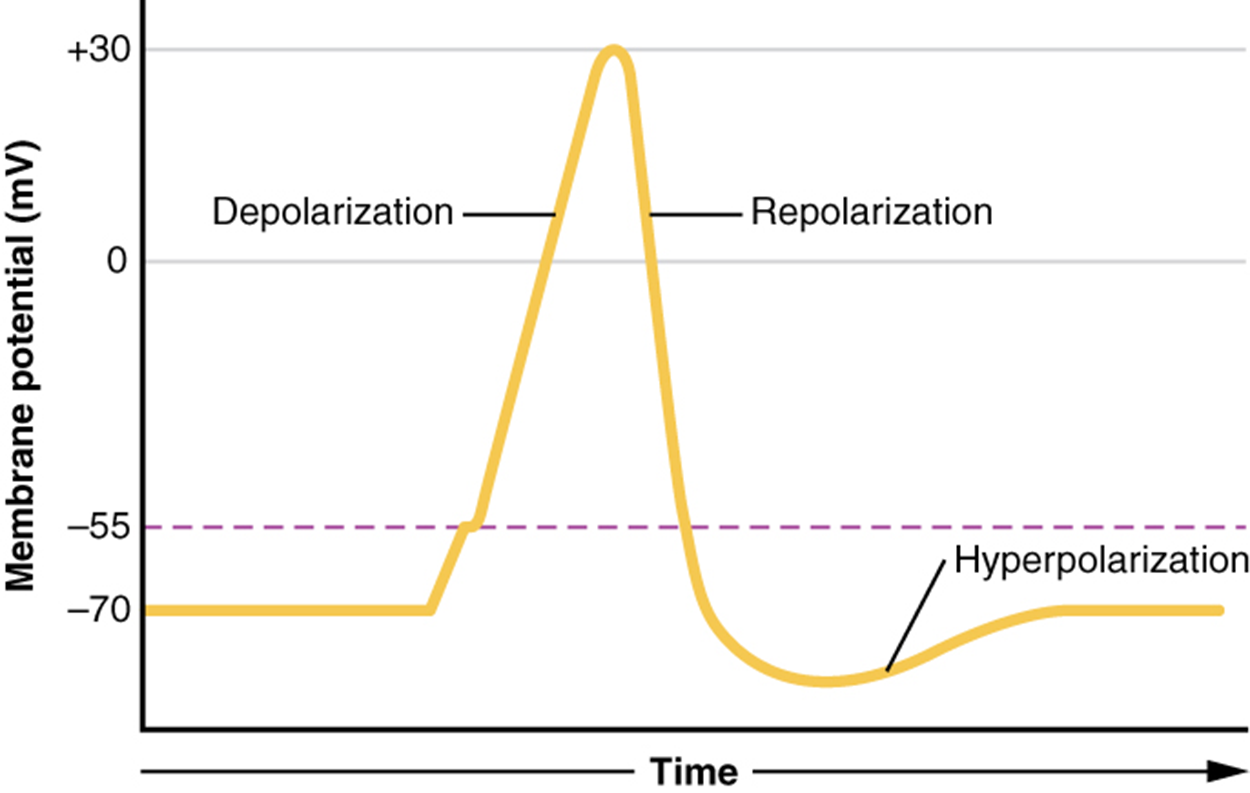
Recall that the concentration of Na⁺ is higher outside the cell. Therefore, when the sodium channel opens, sodium ions rush into the cell. Because sodium is a positively charged ion, it will change the relative voltage inside the cell relative to the outside. The resting potential is the state of the membrane at a voltage of -70 mV, so the sodium cation entering the cell will cause it to become less negative. This is known as depolarization, meaning the membrane potential moves toward zero.
The concentration gradient for Na⁺ is so strong that it will continue to enter the cell even after the membrane potential has become zero so that the voltage immediately around the pore begins to become positive. The membrane potential will reach +30 mV by the time sodium has entered the cell.
As the membrane potential reaches +30 mV, multiple voltage-gated channels respond. The voltage-gated sodium channel that allowed for depolarization of the membrane closes. Additionally, a voltage-gated potassium channel opens.
Recall that the concentration of K⁺ is higher inside the cell. Therefore, when the potassium channel opens, potassium ions rush out of the cell. As K⁺ starts to leave the cell, taking a positive charge with it, the membrane potential begins to move back toward its resting voltage, -70 mV, a process called repolarization.
The membrane potential decreases as potassium moves out of the cell. When it reaches -70 mV, voltage-gated potassium channels begin to close. As they slowly close, the membrane eventually reaches -90 mV, the equilibrium for potassium ions. This period of excessive negative membrane potential is called hyperpolarization. With both voltage-gated sodium and potassium channels closed, the sodium-potassium pump, which has been active the entire time, can finally make progress on moving both ions back to their respective sides. This returns the membrane potential to resting, -70mV, and resets the sodium and potassium channels in preparation for the next electrical signal.
What has been described above are the steps of an action potential, which are presented as a graph of voltage over time in the image below. It is the electrical signal that nervous tissue generates for communication.
Recall that the action potential was initiated by a “stimulus,” but the description did not explain what that was. Physiologically, this stimulus is an event that causes the membrane potential to increase slightly above resting, moving toward zero. Anatomically, it is the opening of a ligand-gated or mechanically-gated sodium channel. A ligand-gated channel opens when a neurotransmitter binds to its receptor. A mechanically-gated sodium channel opens when the membrane of the sensory receptor is physically manipulated (like pressure applied to the skin). In either case, sodium begins to enter the neuron and the membrane potential becomes less negative.
If the stimulus is strong enough to allow the membrane potential to reach -55 mV, voltage-gated sodium channels open. This membrane potential is known as the threshold. When reached, an action potential occurs. If not reached, an action potential does not occur. There is no middle ground. The generation of an action potential is an all-or-none event. Additionally, the events of an action potential are the same every time—the peak is at +30 mV, hyperpolarization reaches +90 mV, and the membrane returns to -70 mV.
As we have seen, the depolarization and repolarization of an action potential are dependent on two types of channels (the voltage-gated Na⁺ channel and the voltage-gated K⁺ channel). The voltage-gated Na⁺ channel actually has two gates. One is the activation gate, which opens when the membrane potential crosses -55 mV. The other gate is the inactivation gate, which closes after a specific period of time—on the order of a fraction of a millisecond. When a cell is at rest, the activation gate is closed and the inactivation gate is open. However, when the threshold is reached, the activation gate opens, allowing Na⁺ to rush into the cell. Timed with the peak of depolarization, the inactivation gate closes. During repolarization, no more sodium can enter the cell. When the membrane potential passes -55 mV again, the activation gate closes. After that, the inactivation gate re-opens, making the channel ready to start the whole process over again. See these two Na⁺ channel gates in the image below.
The voltage-gated K⁺ channel has only one gate, which is sensitive to a membrane voltage of -50 mV. However, it does not open as quickly as the voltage-gated Na⁺ channel does. It might take a fraction of a millisecond for the channel to open once that voltage has been reached. The timing of this coincides exactly with when the Na⁺ flow peaks, so voltage-gated K⁺ channels open just as the voltage-gated Na⁺ channels are being inactivated. As the membrane potential repolarizes and the voltage passes -50 mV again, the channel closes—again, with a little delay. Potassium continues to leave the cell for a short while and the membrane potential becomes more negative, resulting in the hyperpolarizing overshoot. Then the channel closes again and the membrane can return to the resting potential because of the ongoing activity of the non-gated channels and the Na⁺/K⁺ pump.
See the K⁺ channel gate and the two Na⁺ channel gates in action below.
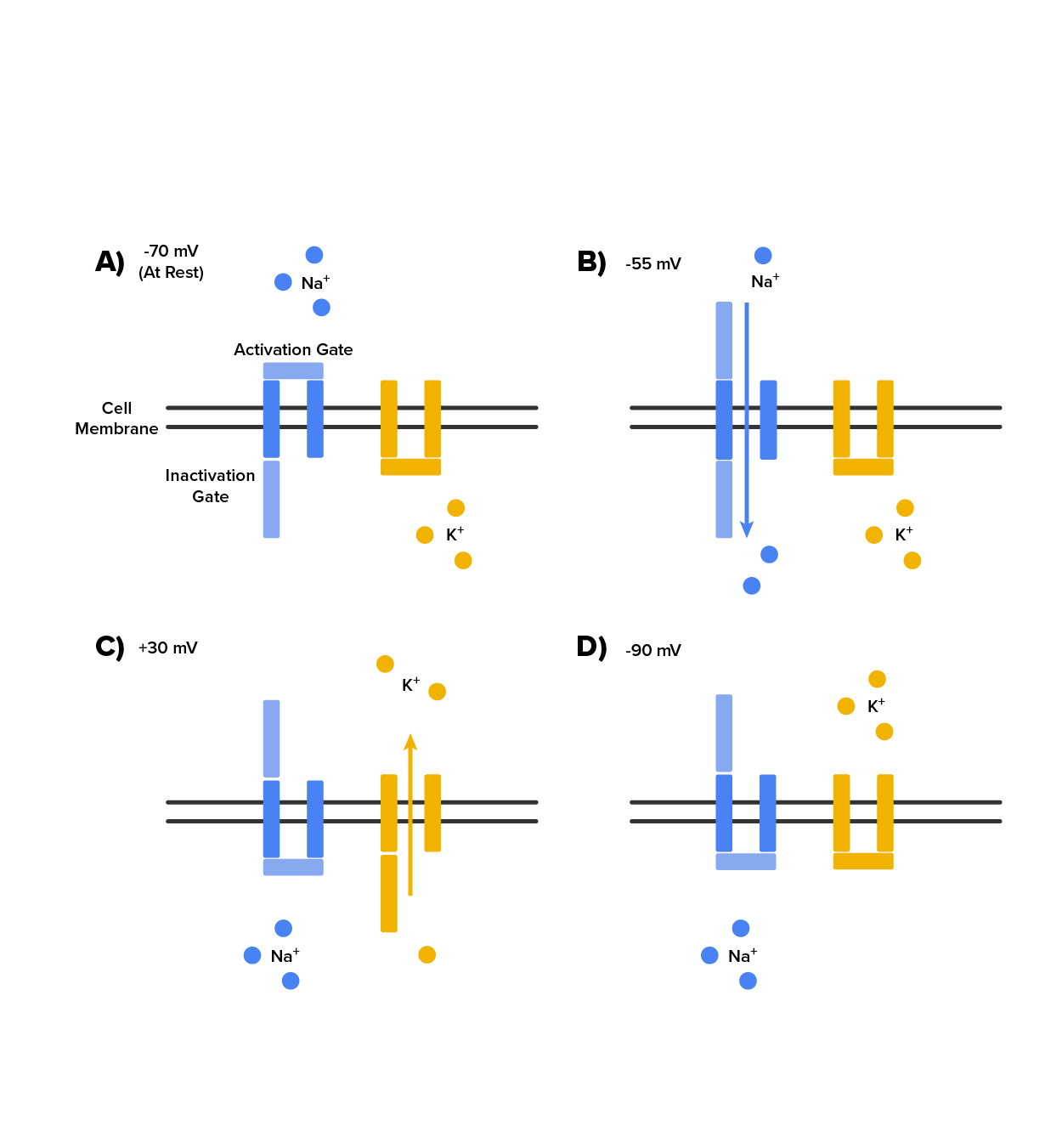
The events of an action potential take place within approximately 2 milliseconds. While an action potential is in progress, another one cannot be initiated. That effect is referred to as the refractory period. There are two phases of the refractory period:
The action potential is initiated at the initial segment, the beginning of the axon. There is a high density of voltage-gated Na⁺ channels in this region so rapid depolarization can take place here. Going down the length of the axon, the action potential is propagated because more voltage-gated Na⁺ channels are opened as the depolarization spreads. This spreading occurs because Na⁺ enters through the channel and moves along the inside of the cell membrane. As the Na⁺ moves, or flows, a short distance along the cell membrane, its positive charge depolarizes a little more of the cell membrane. As that depolarization spreads, new voltage-gated Na⁺ channels open and more ions rush into the cell, spreading the depolarization a little farther.
Because voltage-gated Na⁺ channels are inactivated at the peak of the depolarization, they cannot be opened again for a brief time—the absolute refractory period. Because of this, depolarization spreading back toward previously opened channels has no effect. The action potential must propagate toward the axon terminals; as a result, the polarity of the neuron is maintained, as mentioned above.
In an unmyelinated axon, propagation occurs along the entire length of the axon. This is referred to as continuous propagation. In a myelinated axon, voltage-gated sodium and potassium channels only exist in the nodes of Ranvier. This causes the depolarization to jump from node to node which is called saltatory propagation (saltare, to leap).
Continuous propagation is slow because there are always voltage-gated Na⁺ channels opening, and more and more Na⁺ is rushing into the cell. Saltatory propagation is faster because the action potential jumps from one node to the next, and the new influx of Na⁺ renews the depolarized membrane. Along with the myelination of the axon, the diameter of the axon can influence the speed of conduction. Much as water runs faster in a wide river than in a narrow creek, Na⁺-based depolarization spreads faster down a wide axon than down a narrow one.
IN CONTEXT
Homeostatic Imbalances - Potassium Concentration
Glial cells, especially astrocytes, are responsible for maintaining the chemical environment of the CNS tissue. The concentrations of ions in the extracellular fluid are the basis for how the membrane potential is established and changes in electrochemical signaling. If the balance of ions is upset, drastic outcomes are possible.
Normally the concentration of K⁺ is higher inside the neuron than outside. After the repolarizing phase of the action potential, K⁺ leakage channels and the Na⁺/K⁺ pump ensure that the ions return to their original locations. Following a stroke or other ischemic event, extracellular K⁺ levels are elevated. The astrocytes in the area are equipped to clear excess K⁺ to aid the pump. But when the level is far out of balance, the effects can be irreversible.
Astrocytes can become reactive in cases such as these, which impairs their ability to maintain the local chemical environment. The glial cells enlarge and their processes swell. They lose their K⁺ buffering ability and the function of the pump is affected or even reversed. One of the early signs of cell disease is this "leaking" of sodium ions into the body cells. This sodium/potassium imbalance negatively affects the internal chemistry of cells, preventing them from functioning normally.
Source: THIS CONTENT HAS BEEN ADAPTED FROM OPENSTAX "ANATOMY AND PHYSIOLOGY 2E" AT openstax.org/details/books/anatomy-and-physiology-2e